Naa10p promotes cell invasiveness of esophageal cancer by coordinating the c-Myc and PAI1 regulatory axis
- PMID: 36433943
- PMCID: PMC9700753
- DOI: 10.1038/s41419-022-05441-0
Naa10p promotes cell invasiveness of esophageal cancer by coordinating the c-Myc and PAI1 regulatory axis
Abstract
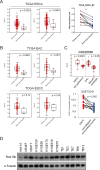
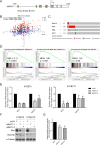
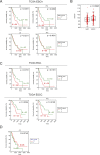
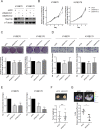
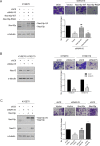
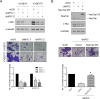
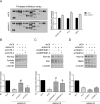
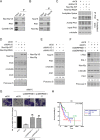
N-α-acetyltransferase 10 protein, Naa10p, is involved in various cellular functions impacting tumor progression. Due to its capacity to acetylate a large spectrum of proteins, both oncogenic and tumor-suppressive roles of Naa10p have been documented. Here, we report an oncogenic role of Naa10p in promoting metastasis of esophageal cancer. NAA10 is more highly expressed in esophageal cancer tissues compared to normal tissues. Higher NAA10 expression also correlates with poorer survival of esophageal cancer patients. We found that NAA10 expression was transcriptionally regulated by the critical oncogene c-Myc in esophageal cancer. Furthermore, activation of the c-Myc-Naa10p axis resulted in upregulated cell invasiveness of esophageal cancer. This increased cell invasiveness was also elucidated to depend on the enzymatic activity of Naa10p. Moreover, Naa10p cooperated with Naa15p to interact with the protease inhibitor, PAI1, and prevent its secretion. This inhibition of PAI1 secretion may derive from the N-terminal acetylation effect of the Naa10p/Naa15p complex. Our results establish the significance of Naa10p in driving metastasis in esophageal cancer by coordinating the c-Myc-PAI1 axis, with implications for its potential use as a prognostic biomarker and therapeutic target for esophageal cancer.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
N-α-acetyltransferase 10 protein promotes metastasis by stabilizing matrix metalloproteinase-2 protein in human osteosarcomas.Cancer Lett. 2018 Oct 1;433:86-98. doi: 10.1016/j.canlet.2018.06.033. Epub 2018 Jun 27. Cancer Lett. 2018. PMID: 29960050
-
Multiple impacts of Naa10p on cancer progression: Molecular functions and clinical prospects.Biochim Biophys Acta Rev Cancer. 2023 Nov;1878(6):188973. doi: 10.1016/j.bbcan.2023.188973. Epub 2023 Aug 31. Biochim Biophys Acta Rev Cancer. 2023. PMID: 37659460 Review.
-
Naa10p Inhibits Beige Adipocyte-Mediated Thermogenesis through N-α-acetylation of Pgc1α.Mol Cell. 2019 Nov 7;76(3):500-515.e8. doi: 10.1016/j.molcel.2019.07.026. Epub 2019 Aug 15. Mol Cell. 2019. PMID: 31422874
-
Stabilization of ADAM9 by N-α-acetyltransferase 10 protein contributes to promoting progression of androgen-independent prostate cancer.Cell Death Dis. 2020 Jul 27;11(7):591. doi: 10.1038/s41419-020-02786-2. Cell Death Dis. 2020. PMID: 32719332 Free PMC article.
-
N-α-acetyltransferase 10 (NAA10) in development: the role of NAA10.Exp Mol Med. 2018 Jul 27;50(7):1-11. doi: 10.1038/s12276-018-0105-2. Exp Mol Med. 2018. PMID: 30054454 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

