Preclinical Evaluation of a Novel Small Molecule Inhibitor of LIM Kinases (LIMK) CEL_Amide in Philadelphia-Chromosome Positive (BCR::ABL+) Acute Lymphoblastic Leukemia (ALL)
- PMID: 36431240
- PMCID: PMC9692768
- DOI: 10.3390/jcm11226761
Preclinical Evaluation of a Novel Small Molecule Inhibitor of LIM Kinases (LIMK) CEL_Amide in Philadelphia-Chromosome Positive (BCR::ABL+) Acute Lymphoblastic Leukemia (ALL)
Abstract
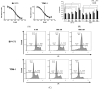
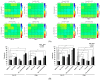
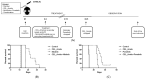
Ph+ (BCR::ABL+) B-ALL was considered to be high risk, but recent advances in BCR::ABL-targeting TKIs has shown improved outcomes in combination with backbone chemotherapy. Nevertheless, new treatment strategies are needed, including approaches without chemotherapy for elderly patients. LIMK1/2 acts downstream from various signaling pathways, which modifies cytoskeleton dynamics via phosphorylation of cofilin. Upstream of LIMK1/2, ROCK is constitutively activated by BCR::ABL, and upon activation, ROCK leads to the phosphorylation of LIMK1/2, resulting in the inactivation of cofilin by its phosphorylation and subsequently abrogating its apoptosis-promoting activity. Here, we demonstrate the anti-leukemic effects of a novel LIMK1/2 inhibitor (LIMKi) CEL_Amide in vitro and in vivo for BCR::ABL-driven B-ALL. The IC50 value of CEL_Amide was ≤1000 nM in BCR::ABL+ TOM-1 and BV-173 cells and induced dose-dependent apoptosis and cell cycle arrest in these cell lines. LIMK1/2 were expressed in BCR::ABL+ cell lines and patient cells and LIMKi treatment decreased LIMK1 protein expression, whereas LIMK2 expression was unaffected. As expected, CEL_Amide exposure caused specific activating downstream dephosphorylation of cofilin in cell lines and primary cells. Combination experiments with CEL_Amide and BCR::ABL TKIs imatinib, dasatinib, nilotinib, and ponatinib were synergistic for the treatment of both TOM-1 and BV-173 cells. CDKN2Ako/BCR::ABL1+ B-ALL cells were transplanted in mice, which were treated with combinations of CEL_Amide and nilotinib or ponatinib, which significantly prolonged their survival. Altogether, the LIMKi CEL_Amide yields activity in Ph+ ALL models when combined with BCR::ABL-targeting TKIs, showing promising synergy that warrants further investigation.
Keywords: ALL; BCR::ABL; CEL_Amide; LIM kinase; tyrosine kinase inhibitors.
Conflict of interest statement
T.B. and H.D. received research funding from CELLIPSE Company. R.P. and F.P. are employed by CELLIPSE company. All other authors have no conflict of interest.
Figures

Similar articles
-
Synergy of FLT3 inhibitors and the small molecule inhibitor of LIM kinase1/2 CEL_Amide in FLT3-ITD mutated Acute Myeloblastic Leukemia (AML) cells.Leuk Res. 2021 Jan;100:106490. doi: 10.1016/j.leukres.2020.106490. Epub 2020 Dec 13. Leuk Res. 2021. PMID: 33373830
-
Combination therapy of BCR-ABL-positive B cell acute lymphoblastic leukemia by tyrosine kinase inhibitor dasatinib and c-JUN N-terminal kinase inhibition.J Hematol Oncol. 2020 Jun 18;13(1):80. doi: 10.1186/s13045-020-00912-3. J Hematol Oncol. 2020. PMID: 32552902 Free PMC article.
-
Tyrosine kinase inhibitors for the treatment of Philadelphia chromosome-positive adult acute lymphoblastic leukemia.Cancer. 2007 Sep 15;110(6):1178-86. doi: 10.1002/cncr.22881. Cancer. 2007. PMID: 17701954 Review.
-
PI3K isoform inhibition associated with anti Bcr-Abl drugs shows in vitro increased anti-leukemic activity in Philadelphia chromosome-positive B-acute lymphoblastic leukemia cell lines.Oncotarget. 2017 Apr 4;8(14):23213-23227. doi: 10.18632/oncotarget.15542. Oncotarget. 2017. PMID: 28390196 Free PMC article.
-
Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia.Clin Ther. 2007 Nov;29(11):2289-308. doi: 10.1016/j.clinthera.2007.11.005. Clin Ther. 2007. PMID: 18158072 Review.
Cited by
-
LIM Kinases, LIMK1 and LIMK2, Are Crucial Node Actors of the Cell Fate: Molecular to Pathological Features.Cells. 2023 Mar 4;12(5):805. doi: 10.3390/cells12050805. Cells. 2023. PMID: 36899941 Free PMC article. Review.
References
-
- Thomas X., Thiebaut A., Olteanu N., Danaila C., Charrin C., Archimbaud E., Fiere D. Philadelphia chromosome positive adult acute lymphoblastic leukemia: Characteristics, prognostic factors and treatment outcome. Hematol. Cell Ther. 1998;40:119–128. - PubMed
-
- Jain N., Maiti A., Ravandi F., Konopleva M., Daver N., Kadia T., Pemmaraju N., Short N., Kebriaei P., Ning J., et al. Inotuzumab ozogamicin with bosutinib for relapsed or refractory Philadelphia chromosome positive acute lymphoblastic leukemia or lymphoid blast phase of chronic myeloid leukemia. Am. J. Hematol. 2021;96:1000–1007. doi: 10.1002/ajh.26238. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

