Neurodegenerative Diseases: From Dysproteostasis, Altered Calcium Signalosome to Selective Neuronal Vulnerability to AAV-Mediated Gene Therapy
- PMID: 36430666
- PMCID: PMC9694178
- DOI: 10.3390/ijms232214188
Neurodegenerative Diseases: From Dysproteostasis, Altered Calcium Signalosome to Selective Neuronal Vulnerability to AAV-Mediated Gene Therapy
Abstract
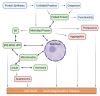
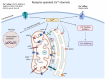
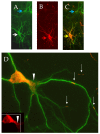
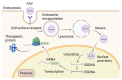
Despite intense research into the multifaceted etiology of neurodegenerative diseases (ND), they remain incurable. Here we provide a brief overview of several major ND and explore novel therapeutic approaches. Although the cause (s) of ND are not fully understood, the accumulation of misfolded/aggregated proteins in the brain is a common pathological feature. This aggregation may initiate disruption of Ca++ signaling, which is an early pathological event leading to altered dendritic structure, neuronal dysfunction, and cell death. Presently, ND gene therapies remain unidimensional, elusive, and limited to modifying one pathological feature while ignoring others. Considering the complexity of signaling cascades in ND, we discuss emerging therapeutic concepts and suggest that deciphering the molecular mechanisms involved in dendritic pathology may broaden the phenotypic spectrum of ND treatment. An innovative multiplexed gene transfer strategy that employs silencing and/or over-expressing multiple effectors could preserve vulnerable neurons before they are lost. Such therapeutic approaches may extend brain health span and ameliorate burdensome chronic disease states.
Keywords: CRMP3/DPYSL4; calcium signaling; dendritic dystrophy; dysproteostasis; gene therapy; neurodegeneration; neuronal vulnerability.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
The dual face of connexin-based astroglial Ca(2+) communication: a key player in brain physiology and a prime target in pathology.Biochim Biophys Acta. 2014 Oct;1843(10):2211-32. doi: 10.1016/j.bbamcr.2014.04.016. Epub 2014 Apr 21. Biochim Biophys Acta. 2014. PMID: 24768716 Review.
-
Iron and ER stress in neurodegenerative disease.Biometals. 2012 Aug;25(4):837-45. doi: 10.1007/s10534-012-9544-8. Epub 2012 Apr 12. Biometals. 2012. PMID: 22526559
-
Cellular pathways leading to neuronal dysfunction and degeneration.Drug News Perspect. 2007 Oct;20(8):501-9. doi: 10.1358/dnp.2007.20.8.1157616. Drug News Perspect. 2007. PMID: 18080037 Review.
-
Molecular insights into mechanisms of the cell death program: role in the progression of neurodegenerative disorders.Curr Alzheimer Res. 2006 Sep;3(4):269-83. doi: 10.2174/156720506778249461. Curr Alzheimer Res. 2006. PMID: 17017859 Review.
-
Role of Endoplasmic Reticulum-Mediated Ca2+ Signaling in Neuronal Cell Death.Antioxid Redox Signal. 2018 Oct 20;29(12):1147-1157. doi: 10.1089/ars.2018.7498. Epub 2018 Mar 14. Antioxid Redox Signal. 2018. PMID: 29361832 Review.
Cited by
-
A Survey on the Expression of the Ubiquitin Proteasome System Components HECT- and RBR-E3 Ubiquitin Ligases and E2 Ubiquitin-Conjugating and E1 Ubiquitin-Activating Enzymes during Human Brain Development.Int J Mol Sci. 2024 Feb 17;25(4):2361. doi: 10.3390/ijms25042361. Int J Mol Sci. 2024. PMID: 38397039 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

