The Renin-Angiotensin-Aldosterone System, Nitric Oxide, and Hydrogen Sulfide at the Crossroads of Hypertension and COVID-19: Racial Disparities and Outcomes
- PMID: 36430371
- PMCID: PMC9699619
- DOI: 10.3390/ijms232213895
The Renin-Angiotensin-Aldosterone System, Nitric Oxide, and Hydrogen Sulfide at the Crossroads of Hypertension and COVID-19: Racial Disparities and Outcomes
Abstract
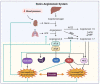
Coronavirus disease 2019 is caused by SARS-CoV-2 and is more severe in the elderly, racial minorities, and those with comorbidities such as hypertension and diabetes. These pathologies are often controlled with medications involving the renin-angiotensin-aldosterone system (RAAS). RAAS is an endocrine system involved in maintaining blood pressure and blood volume through components of the system. SARS-CoV-2 enters the cells through ACE2, a membrane-bound protein related to RAAS. Therefore, the use of RAAS inhibitors could worsen the severity of COVID-19's symptoms, especially amongst those with pre-existing comorbidities. Although a vaccine is currently available to prevent and reduce the symptom severity of COVID-19, other options, such as nitric oxide and hydrogen sulfide, may also have utility to prevent and treat this virus.
Keywords: ACE2; COVID-19; RAAS inhibitors; SARS-CoV-2; health disparities; hydrogen sulfide; hypertension; nitric oxide; renin–angiotensin system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Association of Renin-Angiotensin-Aldosterone Inhibitors with COVID-19 Infection and Disease Severity among Individuals with Hypertension.Isr Med Assoc J. 2022 May;24(5):310-316. Isr Med Assoc J. 2022. PMID: 35598055
-
Serum ACE2, Angiotensin II, and Aldosterone Levels Are Unchanged in Patients With COVID-19.Am J Hypertens. 2021 Apr 2;34(3):278-281. doi: 10.1093/ajh/hpaa169. Am J Hypertens. 2021. PMID: 33043967 Free PMC article.
-
Mechanical dependency of the SARS-CoV-2 virus and the renin-angiotensin-aldosterone (RAAS) axis: a possible new threat.Environ Sci Pollut Res Int. 2022 Sep;29(41):62235-62247. doi: 10.1007/s11356-021-16356-2. Epub 2021 Dec 2. Environ Sci Pollut Res Int. 2022. PMID: 34859345 Free PMC article. Review.
-
Renin-Angiotensin-Aldosterone Inhibitors and COVID-19 Infection.Curr Hypertens Rep. 2022 Oct;24(10):425-433. doi: 10.1007/s11906-022-01207-3. Epub 2022 Jun 18. Curr Hypertens Rep. 2022. PMID: 35716247 Free PMC article. Review.
-
Understanding the renin-angiotensin-aldosterone-SARS-CoV axis: a comprehensive review.Eur Respir J. 2020 Jul 9;56(1):2000912. doi: 10.1183/13993003.00912-2020. Print 2020 Jul. Eur Respir J. 2020. PMID: 32341103 Free PMC article. Review.
Cited by
-
Potential for Early Noninvasive COVID-19 Detection Using Electronic-Nose Technologies and Disease-Specific VOC Metabolic Biomarkers.Sensors (Basel). 2023 Mar 7;23(6):2887. doi: 10.3390/s23062887. Sensors (Basel). 2023. PMID: 36991597 Free PMC article. Review.
-
National and Regional Trends in the Prevalence of Hypertension in South Korea Amid the Pandemic, 2009-2022: Nationwide Study of Over 3 Million Individuals.JMIR Public Health Surveill. 2024 Jul 30;10:e51891. doi: 10.2196/51891. JMIR Public Health Surveill. 2024. PMID: 39078683 Free PMC article.
References
-
- CDC About COVID-19: People with Certain Medical Conditions. [(accessed on 17 August 2022)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

