Mechanism of Resveratrol-Induced Programmed Cell Death and New Drug Discovery against Cancer: A Review
- PMID: 36430164
- PMCID: PMC9697740
- DOI: 10.3390/ijms232213689
Mechanism of Resveratrol-Induced Programmed Cell Death and New Drug Discovery against Cancer: A Review
Abstract
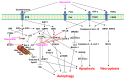
Resveratrol (3,5,4'-trihydroxy-trans-stilbene), a polyphenol found in grapes, red wine, peanuts, and apples, has been reported to exhibit a wide range of biological and pharmacological properties. In addition, resveratrol has been reported to intervene in multiple stages of carcinogenesis. It has also been known to kill several human cancer cells through programmed cell death (PCD) mechanisms such as apoptosis, autophagy, and necroptosis. However, resveratrol has limitations in its use as an anticancer agent because it is susceptible to photoisomerization owing to its unstable double bond, short half-life, and is rapidly metabolized and eliminated. Trans-(E)-resveratrol is nontoxic, and has several biological and pharmacological activities. However, little is known about the pharmacological properties of the photoisomerized cis-(Z)-resveratrol. Therefore, many studies on resveratrol derivatives and analogues that can overcome the shortcomings of resveratrol and increase its anticancer activity are underway. This review comprehensively summarizes the literature related to resveratrol-induced PCD, such as apoptosis, autophagy, necroptosis, and the development status of synthetic resveratrol derivatives and analogues as novel anticancer drugs.
Keywords: apoptosis; autophagy; molecular mechanisms; necroptosis; resveratrol; resveratrol derivatives and analogues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
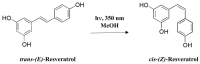

Similar articles
-
Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials.Cancer Prev Res (Phila). 2009 May;2(5):409-18. doi: 10.1158/1940-6207.CAPR-08-0160. Epub 2009 Apr 28. Cancer Prev Res (Phila). 2009. PMID: 19401532 Review.
-
Resveratrol oligomers for the prevention and treatment of cancers.Oxid Med Cell Longev. 2014;2014:765832. doi: 10.1155/2014/765832. Epub 2014 Mar 23. Oxid Med Cell Longev. 2014. PMID: 24799982 Free PMC article. Review.
-
Biological actions and molecular effects of resveratrol, pterostilbene, and 3'-hydroxypterostilbene.J Food Drug Anal. 2017 Jan;25(1):134-147. doi: 10.1016/j.jfda.2016.07.004. Epub 2016 Sep 22. J Food Drug Anal. 2017. PMID: 28911531 Free PMC article. Review.
-
Synthesis and biological evaluation of resveratrol and analogues as apoptosis-inducing agents.J Med Chem. 2003 Jul 31;46(16):3546-54. doi: 10.1021/jm030785u. J Med Chem. 2003. PMID: 12877593
-
Pharmacological activities and structure-modification of resveratrol analogues.Pharmazie. 2015 Dec;70(12):765-71. Pharmazie. 2015. PMID: 26817272 Review.
Cited by
-
Can Resveratrol Influence the Activity of 11β-Hydroxysteroid Dehydrogenase Type 1? A Combined In Silico and In Vivo Study.Pharmaceuticals (Basel). 2023 Feb 7;16(2):251. doi: 10.3390/ph16020251. Pharmaceuticals (Basel). 2023. PMID: 37259398 Free PMC article.
-
Therapeutic effect of targeted antioxidant natural products.Discov Nano. 2024 Sep 10;19(1):144. doi: 10.1186/s11671-024-04100-x. Discov Nano. 2024. PMID: 39251461 Free PMC article. Review.
-
Addition of Polyphenols to Drugs: The Potential of Controlling "Inflammaging" and Fibrosis in Human Senescent Lung Fibroblasts In Vitro.Int J Mol Sci. 2024 Jun 28;25(13):7163. doi: 10.3390/ijms25137163. Int J Mol Sci. 2024. PMID: 39000270 Free PMC article.
-
Systematic Studies on Anti-Cancer Evaluation of Stilbene and Dibenzo[b,f]oxepine Derivatives.Molecules. 2023 Apr 18;28(8):3558. doi: 10.3390/molecules28083558. Molecules. 2023. PMID: 37110792 Free PMC article.
-
Protective and Detoxifying Effects of Resveratrol on Zearalenone-Mediated Toxicity: A Review.Int J Mol Sci. 2024 Oct 13;25(20):11003. doi: 10.3390/ijms252011003. Int J Mol Sci. 2024. PMID: 39456789 Free PMC article. Review.
References
-
- Almatroodi S.A., Alsahli M.A., Aljohani A.S.M., Alhumaydhi F.A., Babiker A.Y., Khan A.A., Rahmani A.H. Potential therapeutic targets of resveratrol, a plant polyphenol, and its role in the therapy of various types of cancer. Molecules. 2022;27:2665. doi: 10.3390/molecules27092665. - DOI - PMC - PubMed
-
- Figueiró F., Bernardi A., Frozza R.L., Terroso T., Zanotto-Filho A., Jandrey E.H., Moreira J.C., Salbego C.G., Edelweiss M.I., Pohlmann A.R., et al. Resveratrol-loaded lipid-core nanocapsules treatment reduces in vitro and in vivo glioma growth. J. Biomed. Nanotechnol. 2013;9:516–526. doi: 10.1166/jbn.2013.1547. - DOI - PubMed
-
- Hu Z., Liang W., Yang Y., Keefe D., Ma Y., Zhao Y., Xue C., Huang Y., Zhao H., Chen L., et al. Personalized estimate of chemotherapy-induced nausea and vomiting: Development and external validation of a nomogram in cancer patients receiving highly/moderately emetogenic chemotherapy. Medicine. 2016;95:e2476. doi: 10.1097/MD.0000000000002476. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

