Antipsychotic Abuse, Dependence, and Withdrawal in the Pediatric Population: A Real-World Disproportionality Analysis
- PMID: 36428541
- PMCID: PMC9687123
- DOI: 10.3390/biomedicines10112972
Antipsychotic Abuse, Dependence, and Withdrawal in the Pediatric Population: A Real-World Disproportionality Analysis
Abstract
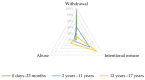
Antipsychotic drugs (APs) aim to treat schizophrenia, bipolar mania, and behavioral symptoms. In child psychiatry, despite limited evidence regarding their efficacy and safety, APs are increasingly subject to off-label use. Studies investigating addictology-related symptoms in young people being scarce, we aimed to characterize the different patterns of AP misuse and withdrawal in children and adolescents relying on the WHO pharmacovigilance database (VigiBase®, Uppsala Monitoring Centre, Sweden). Using the standardized MedDRA Query 'drug abuse, dependence and withdrawal', disproportionality for each AP was assessed with the reporting odds ratio and the information component. A signal was detected when the lower end of the 95% confidence interval of the information component was positive. Results revealed mainly withdrawal symptoms in infants (under 2 years), intentional misuse in children (2 to 11 years), and abuse in adolescents (12 to 17 years). Olanzapine, risperidone, aripiprazole, and quetiapine were disproportionately reported in all age groups, with quetiapine being subject to a specific abuse signal in adolescents. Thus, in adolescents, the evocation of possible recreational consumption may lead to addiction-appropriate care. Further, in young patients with a history of AP treatment, a careful anamnesis may allow one to identify misuse and its role in the case of new-onset symptoms.
Keywords: addictology; adverse drug reaction; antipsychotics; child and adolescent psychiatry; misuse; psychotropic treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Abuse and misuse of second-generation antipsychotics: An analysis using VigiBase, the World Health Organisation pharmacovigilance database.Br J Clin Pharmacol. 2022 Oct;88(10):4646-4653. doi: 10.1111/bcp.15420. Epub 2022 Jun 8. Br J Clin Pharmacol. 2022. PMID: 35633029
-
Drug Class Review: Atypical Antipsychotic Drugs: Final Update 3 Report [Internet].Portland (OR): Oregon Health & Science University; 2010 Jul. Portland (OR): Oregon Health & Science University; 2010 Jul. PMID: 21348048 Free Books & Documents. Review.
-
Cardiac and metabolic safety profile of antipsychotics in youths: A WHO safety database analysis.Psychiatry Res. 2024 Apr;334:115786. doi: 10.1016/j.psychres.2024.115786. Epub 2024 Feb 14. Psychiatry Res. 2024. PMID: 38387164
-
[Antipsychotics in bipolar disorders].Encephale. 2004 Sep-Oct;30(5):417-24. doi: 10.1016/s0013-7006(04)95456-5. Encephale. 2004. PMID: 15627046 Review. French.
-
Weight Gain During Antipsychotic Treatment in Children, Adolescents, and Adults: A Disproportionality Analysis in the Global Pharmacovigilance Database, Vigibase®.Drug Saf. 2023 Jan;46(1):77-85. doi: 10.1007/s40264-022-01252-6. Epub 2022 Dec 2. Drug Saf. 2023. PMID: 36459374
Cited by
-
The Neuropsychiatric Safety Profile of Lasmiditan: A Comparative Disproportionality Analysis with Triptans.Neurotherapeutics. 2023 Sep;20(5):1305-1315. doi: 10.1007/s13311-023-01404-1. Epub 2023 Jul 12. Neurotherapeutics. 2023. PMID: 37436579 Free PMC article.
-
Trends and off-label utilization of antipsychotics in children and adolescents from 2016 to 2021 in China: a real-world study.Child Adolesc Psychiatry Ment Health. 2024 Jun 21;18(1):77. doi: 10.1186/s13034-024-00766-4. Child Adolesc Psychiatry Ment Health. 2024. PMID: 38907356 Free PMC article.
References
-
- Delay J., Deniker P. Chlorpromazine and Neuroleptic Treatments in Psychiatry. J. Clin. Exp. Psychopathol. 1956;17:19–24. - PubMed
-
- Merino D., Fernandez A., Gérard A.O., Ben Othman N., Rocher F., Askenazy F., Verstuyft C., Drici M.-D., Thümmler S. Adverse Drug Reactions of Olanzapine, Clozapine and Loxapine in Children and Youth: A Systematic Pharmacogenetic Review. Pharmaceuticals. 2022;15:749. doi: 10.3390/ph15060749. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

