Safety and immunogenicity of a third dose of COVID-19 protein subunit vaccine (CovovaxTM) after homologous and heterologous two-dose regimens
- PMID: 36427701
- PMCID: PMC9678824
- DOI: 10.1016/j.ijid.2022.11.022
Safety and immunogenicity of a third dose of COVID-19 protein subunit vaccine (CovovaxTM) after homologous and heterologous two-dose regimens
Abstract
Objectives: To report the safety and immunogenicity profile of a protein subunit vaccine (CovovaxTM) given as a third (booster) dose to individuals primed with different primary vaccine regimens.
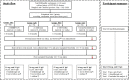
Methods: A third dose was administered to individuals with an interval range of 3-10 months after the second dose. The four groups were classified according to their primary vaccine regimens, including two-dose BBIBP-CorV, AZD1222, BNT162b2, and CoronaVac/AZD1222. Immunogenicity analysis was performed to determine binding antibodies, neutralizing activity, and the T-cell responses.
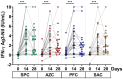
Results: Overall, 210 individuals were enrolled and boosted with the CovovaxTM vaccine. The reactogenicity was mild to moderate. Most participants elicited a high level of binding and neutralizing antibody against Wild-type and Omicron variants after the booster dose. In participants who were antinucleocapsid immunoglobulin G-negative from all groups, a booster dose could elicit neutralizing activity to Wild-type and Omicron variants by more than 95% and 70% inhibition at 28 days, respectively. The CovovaxTM vaccine could elicit a cell-mediated immune response.
Conclusion: The protein subunit vaccine (CovovaxTM) can be proposed as a booster dose after two different priming dose regimens. It has strong immunogenicity and good safety profiles.
Keywords: Booster dose; Covovax(TM); Novavax; Omicron; SARS-CoV-2; Side effect.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no competing interests to declare.
Figures



Similar articles
-
Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study.Lancet. 2022 Feb 5;399(10324):521-529. doi: 10.1016/S0140-6736(22)00094-0. Epub 2022 Jan 21. Lancet. 2022. PMID: 35074136 Free PMC article. Clinical Trial.
-
Immunogenicity and reactogenicity against the SARS-CoV-2 variants following heterologous primary series involving CoronaVac, ChAdox1 nCov-19 and BNT162b2 plus BNT162b2 booster vaccination: An open-label randomized study in healthy Thai adults.Hum Vaccin Immunother. 2022 Nov 30;18(6):2091865. doi: 10.1080/21645515.2022.2091865. Epub 2022 Jul 11. Hum Vaccin Immunother. 2022. PMID: 35816053 Free PMC article. Clinical Trial.
-
Immunogenicity and safety in healthy adults of full dose versus half doses of COVID-19 vaccine (ChAdOx1-S or BNT162b2) or full-dose CoronaVac administered as a booster dose after priming with CoronaVac: a randomised, observer-masked, controlled trial in Indonesia.Lancet Infect Dis. 2023 May;23(5):545-555. doi: 10.1016/S1473-3099(22)00800-3. Epub 2023 Jan 11. Lancet Infect Dis. 2023. PMID: 36640798 Clinical Trial.
-
Safety and immunogenicity of homologous versus heterologous booster dose with AZD1222, mRNA-1273, or MVC-COV1901 SARS-CoV-2 vaccines in adults: An observer-blinded, multi-center, phase 2 randomized trial.Vaccine. 2023 May 26;41(23):3497-3505. doi: 10.1016/j.vaccine.2023.04.029. Epub 2023 Apr 12. Vaccine. 2023. PMID: 37080829 Free PMC article. Clinical Trial.
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article. Review.
Cited by
-
Structural understanding of SARS-CoV-2 virus entry to host cells.Front Mol Biosci. 2023 Nov 2;10:1288686. doi: 10.3389/fmolb.2023.1288686. eCollection 2023. Front Mol Biosci. 2023. PMID: 38033388 Free PMC article. Review.
-
Editorial: COVID-19 booster vaccination: increasing immunity against life-threatening infection.Front Public Health. 2024 Jan 9;11:1342118. doi: 10.3389/fpubh.2023.1342118. eCollection 2023. Front Public Health. 2024. PMID: 38264241 Free PMC article. No abstract available.
-
The Fourth Dose of mRNA COVID-19 Vaccine Following 12 Different Three-Dose Regimens: Safety and Immunogenicity to Omicron BA.4/BA.5.Vaccines (Basel). 2023 Mar 1;11(3):570. doi: 10.3390/vaccines11030570. Vaccines (Basel). 2023. PMID: 36992154 Free PMC article.
-
Immunogenicity of NVX-CoV2373 heterologous boost against SARS-CoV-2 variants.NPJ Vaccines. 2023 Jul 11;8(1):98. doi: 10.1038/s41541-023-00693-z. NPJ Vaccines. 2023. PMID: 37433788 Free PMC article.
-
Evaluation of Anti-S1 IgA Response to Different COVID-19 Vaccination Regimens.Vaccines (Basel). 2023 Jun 19;11(6):1117. doi: 10.3390/vaccines11061117. Vaccines (Basel). 2023. PMID: 37376506 Free PMC article.
References
-
- Assawakosri S, Kanokudom S, Chansaenroj J, Suntronwong N, Auphimai C, Nilyanimit P, et al. Persistence of immunity against Omicron BA.1 and BA.2 variants following homologous and heterologous COVID-19 booster vaccines in healthy adults after a two-dose AZD1222 vaccination. Int J Infect Dis. 2022;122:793–801. - PMC - PubMed
-
- Assawakosri S, Kanokudom S, Suntronwong N, Auphimai C, Nilyanimit P, Vichaiwattana P, et al. Neutralizing activities against the omicron variant after a heterologous booster in healthy adults receiving two doses of CoronaVac vaccination. J Infect Dis. 2022;226:1372–1381. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

