Response of root and root hair phenotypes of cotton seedlings under high temperature revealed with RhizoPot
- PMID: 36426149
- PMCID: PMC9679381
- DOI: 10.3389/fpls.2022.1007145
Response of root and root hair phenotypes of cotton seedlings under high temperature revealed with RhizoPot
Abstract
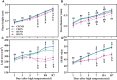
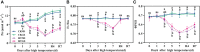
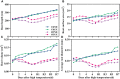
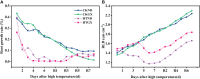
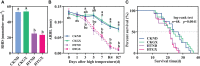
Driven by the increase in its frequency and duration, high temperature weather is increasingly seriously affecting crop development. High temperature inhibits the leaf development, flowering, and pollination of cotton, but its effects on the roots and root hair phenotypes and lifespans remain unclear. Thus, this study selected the two cotton varieties Nongda 601 (ND) and Guoxin 9 (GX) as materials and adopted the RhizoPot, an in situ root observation system, to investigate the effects of high temperature (38°C day and 32°C night) on the growth dynamics of the aboveground parts and root phenotypes of cotton at the seedling stage. The results showed that high temperature reduced the net photosynthetic rate and chlorophyll content, decreased the dry matter accumulation and transfer to the root, and lowered the root-shoot ratio (R/S ratio). The root phenotypes changed significantly under high temperature. After 7 d of high temperature stress, the root lengths of ND and GX decreased by 78.14 mm and 59.64 mm, respectively. Their specific root lengths increased by 79.60% and 66.11%, respectively. Their specific root surface areas increased by 418.70 cm2·g-1 and 433.42 cm2·g-1, respectively. Their proportions of very fine roots increased to 99.26% and 97.16%, respectively. After the removal of high temperature (RHT), their root lengths tended to increase, and their proportions of very fine roots continued to increase. The root hairs of ND and GX were also significantly affected by high temperature. In particular, the root hair densities of ND and GX decreased by 52.53% and 56.25%, respectively. Their average root hair lengths decreased by 96.62% and 74.29%, respectively. Their root hair lifespans decreased by 7 d and 10 d, respectively. After the RHT, their average root hair lengths failed to recover. A principal component analysis indicated that the root architectures were significantly affected by root hair density, average root hair length, specific root length, and specific root surface area under high temperatures. In summary, cotton adapts to high temperature environments by increasing the specific root length, specific root surface area, and the proportions of very fine roots, and reducing the lifespan of root hairs.
Keywords: RhizoPot; cotton; high temperature; in situ root; root dynamics; root hair.
Copyright © 2022 Fan, Hou, Si, Sun, Zhang, Bai, Wang, Li, Liu and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Response of in situ root phenotypes to potassium stress in cotton.PeerJ. 2023 Jun 21;11:e15587. doi: 10.7717/peerj.15587. eCollection 2023. PeerJ. 2023. PMID: 37361035 Free PMC article.
-
In situ Root Phenotypes of Cotton Seedlings Under Phosphorus Stress Revealed Through RhizoPot.Front Plant Sci. 2021 Aug 30;12:716691. doi: 10.3389/fpls.2021.716691. eCollection 2021. Front Plant Sci. 2021. PMID: 34527012 Free PMC article.
-
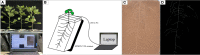
RhizoPot platform: A high-throughput in situ root phenotyping platform with integrated hardware and software.Front Plant Sci. 2022 Sep 29;13:1004904. doi: 10.3389/fpls.2022.1004904. eCollection 2022. Front Plant Sci. 2022. PMID: 36247541 Free PMC article.
-
Salinity-induced reduction in root surface area and changes in major root and shoot traits at the phytomer level in wheat.J Exp Bot. 2016 Jun;67(12):3719-29. doi: 10.1093/jxb/erw064. Epub 2016 Mar 7. J Exp Bot. 2016. PMID: 26951370
-
[Responses of main characters of root system to salt stress among cotton varieties with diffe-rent salt tolerance].Ying Yong Sheng Tai Xue Bao. 2018 Mar;29(3):865-873. doi: 10.13287/j.1001-9332.201803.025. Ying Yong Sheng Tai Xue Bao. 2018. PMID: 29722229 Chinese.
Cited by
-
Plants and global warming: challenges and strategies for a warming world.Plant Cell Rep. 2024 Jan 2;43(1):27. doi: 10.1007/s00299-023-03083-w. Plant Cell Rep. 2024. PMID: 38163826 Review.
-
Whole-Genome Bisulfite Sequencing (WGBS) Analysis of Gossypium hirsutum under High-Temperature Stress Conditions.Genes (Basel). 2024 Sep 24;15(10):1241. doi: 10.3390/genes15101241. Genes (Basel). 2024. PMID: 39457365 Free PMC article.
-
Response of in situ root phenotypes to potassium stress in cotton.PeerJ. 2023 Jun 21;11:e15587. doi: 10.7717/peerj.15587. eCollection 2023. PeerJ. 2023. PMID: 37361035 Free PMC article.
References
-
- Alsajri F. A., Singh B., Wijewardana C., Irby J. T., Gao W., Reddy K. R. (2019). Evaluating soybean cultivars for low-and high-temperature tolerance during the seedling growth stage. Agronomy 9, 13. doi: 10.3390/agronomy9010013 - DOI
-
- Bates T. R., Lynch J. P. (2001). Root hairs confer a competitive advantage under low phosphorus availability. Plant Soil 236, 243–250. doi: 10.1023/A:1012791706800 - DOI
-
- Benlloch-Gonzalez M., Bochicchio R., Berger J., Bramley H., Palta J. A. (2014). High temperature reduces the positive effect of elevated CO2 on wheat root system growth. Field Crops Res. 165, 71–79. doi: 10.1016/j.fcr.2014.04.008 - DOI
LinkOut - more resources
Full Text Sources

