Signaling pathways in cancer-associated fibroblasts: recent advances and future perspectives
- PMID: 36424360
- PMCID: PMC9859735
- DOI: 10.1002/cac2.12392
Signaling pathways in cancer-associated fibroblasts: recent advances and future perspectives
Abstract
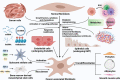
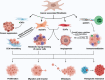
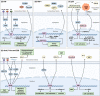
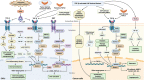
As a critical component of the tumor microenvironment (TME), cancer-associated fibroblasts (CAFs) play important roles in cancer initiation and progression. Well-known signaling pathways, including the transforming growth factor-β (TGF-β), Hedgehog (Hh), Notch, Wnt, Hippo, nuclear factor kappa-B (NF-κB), Janus kinase (JAK)/signal transducer and activator of transcription (STAT), mitogen-activated protein kinase (MAPK), and phosphoinositide 3-kinase (PI3K)/AKT pathways, as well as transcription factors, including hypoxia-inducible factor (HIF), heat shock transcription factor 1 (HSF1), P53, Snail, and Twist, constitute complex regulatory networks in the TME to modulate the formation, activation, heterogeneity, metabolic characteristics and malignant phenotype of CAFs. Activated CAFs remodel the TME and influence the malignant biological processes of cancer cells by altering the transcriptional and secretory characteristics, and this modulation partially depends on the regulation of signaling cascades. The results of preclinical and clinical trials indicated that therapies targeting signaling pathways in CAFs demonstrated promising efficacy but were also accompanied by some failures (e.g., NCT01130142 and NCT01064622). Hence, a comprehensive understanding of the signaling cascades in CAFs might help us better understand the roles of CAFs and the TME in cancer progression and may facilitate the development of more efficient and safer stroma-targeted cancer therapies. Here, we review recent advances in studies of signaling pathways in CAFs and briefly discuss some future perspectives on CAF research.
Keywords: Cancer-associated fibroblasts; Cell-cell interaction; Signaling pathways; Therapeutic targets; Tumor microenvironment.
© 2022 The Authors. Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat-sen University Cancer Center.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer.Signal Transduct Target Ther. 2021 Jun 10;6(1):218. doi: 10.1038/s41392-021-00641-0. Signal Transduct Target Ther. 2021. PMID: 34108441 Free PMC article. Review.
-
Regulation of heterogeneous cancer-associated fibroblasts: the molecular pathology of activated signaling pathways.J Exp Clin Cancer Res. 2020 Jun 16;39(1):112. doi: 10.1186/s13046-020-01611-0. J Exp Clin Cancer Res. 2020. PMID: 32546182 Free PMC article. Review.
-
Architecture of Cancer-Associated Fibroblasts in Tumor Microenvironment: Mapping Their Origins, Heterogeneity, and Role in Cancer Therapy Resistance.OMICS. 2020 Jun;24(6):314-339. doi: 10.1089/omi.2020.0023. OMICS. 2020. PMID: 32496970
-
Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives.Mol Cancer. 2021 Oct 11;20(1):131. doi: 10.1186/s12943-021-01428-1. Mol Cancer. 2021. PMID: 34635121 Free PMC article. Review.
-
Adipose-Derived Stromal Cells and Cancer-Associated Fibroblasts: Interactions and Implications in Tumor Progression.Int J Mol Sci. 2024 Oct 28;25(21):11558. doi: 10.3390/ijms252111558. Int J Mol Sci. 2024. PMID: 39519109 Free PMC article. Review.
Cited by
-
Anticancer activities of natural antimicrobial peptides from animals.Front Microbiol. 2024 Jan 17;14:1321386. doi: 10.3389/fmicb.2023.1321386. eCollection 2023. Front Microbiol. 2024. PMID: 38298540 Free PMC article. Review.
-
Tinosporae Radix attenuates acute pharyngitis by regulating glycerophospholipid metabolism and inflammatory responses through PI3K-Akt signaling pathway.Front Pharmacol. 2024 Nov 6;15:1491321. doi: 10.3389/fphar.2024.1491321. eCollection 2024. Front Pharmacol. 2024. PMID: 39568590 Free PMC article.
-
Spheroids of FAP-Positive Cell Lines as a Model for Screening Drugs That Affect FAP Expression.Biomedicines. 2023 Jul 18;11(7):2017. doi: 10.3390/biomedicines11072017. Biomedicines. 2023. PMID: 37509656 Free PMC article.
-
Is Insulin Receptor Substrate4 (IRS4) a Platform Involved in the Activation of Several Oncogenes?Cancers (Basel). 2023 Sep 20;15(18):4651. doi: 10.3390/cancers15184651. Cancers (Basel). 2023. PMID: 37760618 Free PMC article. Review.
-
The role of noncoding RNAs in the tumor microenvironment of hepatocellular carcinoma.Acta Biochim Biophys Sin (Shanghai). 2023 Nov 25;55(11):1697-1706. doi: 10.3724/abbs.2023231. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 37867435 Free PMC article. Review.
References
-
- Junttila MR, de Sauvage FJ. Influence of tumour micro‐environment heterogeneity on therapeutic response. Nature. 2013;501:346–54. - PubMed
-
- Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer‐associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev. 2016;99:186–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81802352/National Natural Science Foundation of China
- 82002541/National Natural Science Foundation of China
- 81772555/National Natural Science Foundation of China
- 81902428/National Natural Science Foundation of China
- 81625016/National Science Foundation for Distinguished Young Scholars of China
- 20YF1409000/Shanghai Sailing Program
- 20QA1402100/Shanghai Rising-Star Program
- SACA-CY19A06/Shanghai Anticancer Association Young Eagle Program
- SHDC12018109/Clinical and Scientific Innovation Project of Shanghai Hospital Development Center
- SHDC12019109/Clinical and Scientific Innovation Project of Shanghai Hospital Development Center
- 2019-01-07-00-07-E00057/Scientific Innovation Project of Shanghai Education Committee
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

