Characterization of Neutralizing Monoclonal Antibodies and Identification of a Novel Conserved C-Terminal Linear Epitope on the Hemagglutinin Protein of the H9N2 Avian Influenza Virus
- PMID: 36423139
- PMCID: PMC9698441
- DOI: 10.3390/v14112530
Characterization of Neutralizing Monoclonal Antibodies and Identification of a Novel Conserved C-Terminal Linear Epitope on the Hemagglutinin Protein of the H9N2 Avian Influenza Virus
Abstract
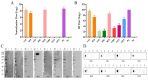
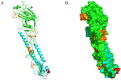
The H9N2 avian influenza virus (AIV) remains a serious threat to the global poultry industry and public health. The hemagglutinin (HA) protein is an essential protective antigen of AIVs and a major target of neutralizing antibodies and vaccines. Therefore, in this study, we used rice-derived HA protein as an immunogen to generate monoclonal antibodies (mAbs) and screened them using an immunoperoxidase monolayer assay and indirect enzyme-linked immunosorbent assay. Eight mAbs reacted well with the recombinant H9N2 AIV and HA protein, four of which exhibited potent inhibitory activity against hemagglutination, while three showed remarkable neutralization capacities. Western blotting confirmed that two mAbs bound to the HA protein. Linear epitopes were identified using the mAbs; a novel linear epitope, 480HKCDDQCM487, was identified. Structural analysis revealed that the novel linear epitope is located at the C-terminus of HA2 near the disulfide bond-linked HA1 and HA2. Alignment of the amino acid sequences showed that the epitope was highly conserved among multiple H9N2 AIV strains. The results of this study provide novel insights for refining vaccine and diagnostic strategies and expand our understanding of the immune response against AIV.
Keywords: H9N2 avian influenza virus; HA2; hemagglutinin protein; linear B cell epitope; neutralizing monoclonal antibodies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Identification of Unique and Conserved Neutralizing Epitopes of Vestigial Esterase Domain in HA Protein of the H9N2 Subtype of Avian Influenza Virus.Viruses. 2022 Dec 8;14(12):2739. doi: 10.3390/v14122739. Viruses. 2022. PMID: 36560743 Free PMC article.
-
Vaccine Efficacy of Inactivated, Chimeric Hemagglutinin H9/H5N2 Avian Influenza Virus and Its Suitability for the Marker Vaccine Strategy.J Virol. 2017 Feb 28;91(6):e01693-16. doi: 10.1128/JVI.01693-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077631 Free PMC article.
-
Identification of catalytically active domain epitopes in neuraminidase protein of H9N2 subtype of avian influenza virus.Avian Pathol. 2023 Oct;52(5):377-387. doi: 10.1080/03079457.2023.2239191. Epub 2023 Aug 15. Avian Pathol. 2023. PMID: 37581283
-
B cell epitopes in the intrinsically disordered regions of neuraminidase and hemagglutinin proteins of H5N1 and H9N2 avian influenza viruses for peptide-based vaccine development.J Cell Biochem. 2019 Oct;120(10):17534-17544. doi: 10.1002/jcb.29017. Epub 2019 May 20. J Cell Biochem. 2019. PMID: 31111560
-
Analysis of the conserved protective epitopes of hemagglutinin on influenza A viruses.Front Immunol. 2023 Feb 17;14:1086297. doi: 10.3389/fimmu.2023.1086297. eCollection 2023. Front Immunol. 2023. PMID: 36875062 Free PMC article. Review.
References
-
- Eladl A.H., Mosad S.M., El-Shafei R.A., Saleh R.M., Ali H.S., Badawy B.M., Elshal M.F. Immunostimulant effect of a mixed herbal extract on infectious bursal disease virus (IBDV) vaccinated chickens in the context of a co-infection model of avian influenza virus H9N2 and IBDV. Comp. Immunol. Microbiol. Infect. Dis. 2020;72:101505. doi: 10.1016/j.cimid.2020.101505. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

