Superantigens, a Paradox of the Immune Response
- PMID: 36422975
- PMCID: PMC9692936
- DOI: 10.3390/toxins14110800
Superantigens, a Paradox of the Immune Response
Abstract
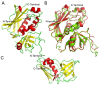
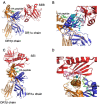
Staphylococcal enterotoxins are a wide family of bacterial exotoxins with the capacity to activate as much as 20% of the host T cells, which is why they were called superantigens. Superantigens (SAgs) can cause multiple diseases in humans and cattle, ranging from mild to life-threatening infections. Almost all S. aureus isolates encode at least one of these toxins, though there is no complete knowledge about how their production is triggered. One of the main problems with the available evidence for these toxins is that most studies have been conducted with a few superantigens; however, the resulting characteristics are attributed to the whole group. Although these toxins share homology and a two-domain structure organization, the similarity ratio varies from 20 to 89% among different SAgs, implying wide heterogeneity. Furthermore, every attempt to structurally classify these proteins has failed to answer differential biological functionalities. Taking these concerns into account, it might not be appropriate to extrapolate all the information that is currently available to every staphylococcal SAg. Here, we aimed to gather the available information about all staphylococcal SAgs, considering their functions and pathogenicity, their ability to interact with the immune system as well as their capacity to be used as immunotherapeutic agents, resembling the two faces of Dr. Jekyll and Mr. Hyde.
Keywords: enterotoxin; immunomodulation; molecular and cellular targets; staphylococcal superantigen; toxin pathogenicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
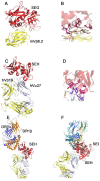
Similar articles
-
Population Analysis of Staphylococcus aureus Reveals a Cryptic, Highly Prevalent Superantigen SElW That Contributes to the Pathogenesis of Bacteremia.mBio. 2020 Oct 27;11(5):e02082-20. doi: 10.1128/mBio.02082-20. mBio. 2020. PMID: 33109757 Free PMC article.
-
Molecular analysis of staphylococcal superantigens.Methods Mol Biol. 2014;1085:169-85. doi: 10.1007/978-1-62703-664-1_10. Methods Mol Biol. 2014. PMID: 24085696
-
Staphylococcal superantigens in colonization and disease.Front Cell Infect Microbiol. 2012 Apr 17;2:52. doi: 10.3389/fcimb.2012.00052. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919643 Free PMC article. Review.
-
Superantigens promote Staphylococcus aureus bloodstream infection by eliciting pathogenic interferon-gamma production.Proc Natl Acad Sci U S A. 2022 Feb 22;119(8):e2115987119. doi: 10.1073/pnas.2115987119. Proc Natl Acad Sci U S A. 2022. PMID: 35165181 Free PMC article.
-
Staphylococcal superantigens interact with multiple host receptors to cause serious diseases.Immunol Res. 2014 Aug;59(1-3):177-81. doi: 10.1007/s12026-014-8539-7. Immunol Res. 2014. PMID: 24838262 Free PMC article. Review.
Cited by
-
Editorial of the Special Issue "Toxins: Mr Hyde or Dr Jekyll?".Toxins (Basel). 2023 Feb 10;15(2):142. doi: 10.3390/toxins15020142. Toxins (Basel). 2023. PMID: 36828456 Free PMC article.
-
Staphylococcal Enterotoxins: Description and Importance in Food.Pathogens. 2024 Aug 9;13(8):676. doi: 10.3390/pathogens13080676. Pathogens. 2024. PMID: 39204276 Free PMC article. Review.
-
Exploring HERV-K (HML-2) Influence in Cancer and Prospects for Therapeutic Interventions.Int J Mol Sci. 2023 Sep 27;24(19):14631. doi: 10.3390/ijms241914631. Int J Mol Sci. 2023. PMID: 37834078 Free PMC article. Review.
-
Toxic Shock Syndrome: A Literature Review.Antibiotics (Basel). 2024 Jan 18;13(1):96. doi: 10.3390/antibiotics13010096. Antibiotics (Basel). 2024. PMID: 38247655 Free PMC article. Review.
-
Current Views about the Inflammatory Damage Triggered by Bacterial Superantigens and Experimental Attempts to Neutralize Superantigen-Mediated Toxic Effects with Natural and Biological Products.Pathophysiology. 2024 Jan 9;31(1):18-31. doi: 10.3390/pathophysiology31010002. Pathophysiology. 2024. PMID: 38251046 Free PMC article. Review.
References
-
- Schutzer S.E., Fischetti V.A., Zabriskie J.B. Toxic Shock Syndrome and Lysogeny in Staphylococcus aureus. Obstet. Gynecol. Surv. 1983;220:316–318. - PubMed
-
- Gaventa S., Reingold A.L., Hightower A.W., Broome C.V., Schwartz B., Hoppe C., Harwell J., Lefkowitz L.K., Makintubee S., Cundiff D.R., et al. Active surveillance for toxic shock syndrome in the united states, 1986. Rev. Infect. Dis. 1989;11:S28–S34. doi: 10.1093/clinids/11.Supplement_1.S28. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

