A Physiologically Based Pharmacokinetic (PBPK) Modeling Framework for Mixtures of Dioxin-like Compounds
- PMID: 36422908
- PMCID: PMC9698634
- DOI: 10.3390/toxics10110700
A Physiologically Based Pharmacokinetic (PBPK) Modeling Framework for Mixtures of Dioxin-like Compounds
Abstract
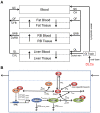
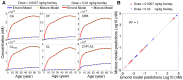
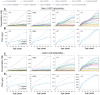
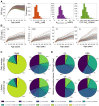
Humans are exposed to persistent organic pollutants, such as dioxin-like compounds (DLCs), as mixtures. Understanding and predicting the toxicokinetics and thus internal burden of major constituents of a DLC mixture is important for assessing their contributions to health risks. PBPK models, including dioxin models, traditionally focus on one or a small number of compounds; developing new or extending existing models for mixtures often requires tedious, error-prone coding work. This lack of efficiency to scale up for multi-compound exposures is a major technical barrier toward large-scale mixture PBPK simulations. Congeners in the DLC family, including 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), share similar albeit quantitatively different toxicokinetic and toxicodynamic properties. Taking advantage of these similarities, here we reported the development of a human PBPK modeling framework for DLC mixtures that can flexibly accommodate an arbitrary number of congeners. Adapted from existing TCDD models, our mixture model contains the blood and three diffusion-limited compartments-liver, fat, and rest of the body. Depending on the number of congeners in a mixture, varying-length vectors of ordinary differential equations (ODEs) are automatically generated to track the tissue concentrations of the congeners. Shared ODEs are used to account for common variables, including the aryl hydrocarbon receptor (AHR) and CYP1A2, to which the congeners compete for binding. Binary and multi-congener mixture simulations showed that the AHR-mediated cross-induction of CYP1A2 accelerates the sequestration and metabolism of DLC congeners, resulting in consistently lower tissue burdens than in single exposure, except for the liver. Using dietary intake data to simulate lifetime exposures to DLC mixtures, the model demonstrated that the relative contributions of individual congeners to blood or tissue toxic equivalency (TEQ) values are markedly different than those to intake TEQ. In summary, we developed a mixture PBPK modeling framework for DLCs that may be utilized upon further improvement as a quantitative tool to estimate tissue dosimetry and health risks of DLC mixtures.
Keywords: AHR; PBPK; TCDD; TEQ; dioxin-like compounds; mixture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Toxicology and carcinogenesis studies of a mixture of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Cas No. 1746-01-6), 2,3,4,7,8-pentachlorodibenzofuran (PeCDF) (Cas No. 57117-31-4), and 3,3',4,4',5-pentachlorobiphenyl (PCB 126) (Cas No. 57465-28-8) in female Harlan Sprague-Dawley rats (gavage studies).Natl Toxicol Program Tech Rep Ser. 2006 Sep;(526):1-180. Natl Toxicol Program Tech Rep Ser. 2006. PMID: 17342195
-
Toxicology and carcinogenesis studies of a binary mixture of 3,3',4,4',5-pentachlorobiphenyl (PCB 126) (Cas No. 57465-28-8) and 2,3',4,4',5-pentachlorobiphenyl (PCB 118) (Cas No. 31508-00-6) in female Harlan Sprague-Dawley rats (gavage studies).Natl Toxicol Program Tech Rep Ser. 2006 Nov;(531):1-218. Natl Toxicol Program Tech Rep Ser. 2006. PMID: 17342196
-
NTP technical report on the toxicology and carcinogenesis studies of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (CAS No. 1746-01-6) in female Harlan Sprague-Dawley rats (Gavage Studies).Natl Toxicol Program Tech Rep Ser. 2006 Apr;(521):4-232. Natl Toxicol Program Tech Rep Ser. 2006. PMID: 16835633
-
A review of background dioxin concentrations in urban/suburban and rural soils across the United States: implications for site assessments and the establishment of soil cleanup levels.Sci Total Environ. 2014 Jan 1;466-467:586-97. doi: 10.1016/j.scitotenv.2013.07.065. Epub 2013 Aug 15. Sci Total Environ. 2014. PMID: 23955251 Review.
-
Development validation and problems with the toxic equivalency factor approach for risk assessment of dioxins and related compounds.J Anim Sci. 1998 Jan;76(1):134-41. doi: 10.2527/1998.761134x. J Anim Sci. 1998. PMID: 9464894 Review.
Cited by
-
Consequences of reprogramming acetyl-CoA metabolism by 2,3,7,8-tetrachlorodibenzo-p-dioxin in the mouse liver.Sci Rep. 2023 Mar 13;13(1):4138. doi: 10.1038/s41598-023-31087-9. Sci Rep. 2023. PMID: 36914879 Free PMC article.
-
Endocrine Disruptor Compounds in Environment: Focus on Women's Reproductive Health and Endometriosis.Int J Mol Sci. 2023 Mar 16;24(6):5682. doi: 10.3390/ijms24065682. Int J Mol Sci. 2023. PMID: 36982755 Free PMC article. Review.
-
Hepatic cholesterol biosynthesis and dioxin-induced dysregulation: A multiscale computational approach.Food Chem Toxicol. 2023 Nov;181:114086. doi: 10.1016/j.fct.2023.114086. Epub 2023 Oct 10. Food Chem Toxicol. 2023. PMID: 37820785 Free PMC article.
References
-
- Van den Berg M., Birnbaum L.S., Denison M., De Vito M., Farland W., Feeley M., Fiedler H., Hakansson H., Hanberg A., Haws L., et al. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. - DOI - PMC - PubMed
-
- ATSDR . Toxicological Profile for Chlorinated Dibenzo-p-Dioxins (CDDs) U.S. Department of Health and Human Services, P.H.S.; Atlanta, GA, USA: 1998. [(accessed on 14 November 2022)]. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp104.pdf.
Grants and funding
LinkOut - more resources
Full Text Sources

