New Antifungal Agents with Azole Moieties
- PMID: 36422557
- PMCID: PMC9698508
- DOI: 10.3390/ph15111427
New Antifungal Agents with Azole Moieties
Abstract
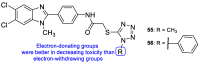
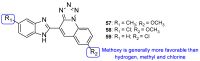
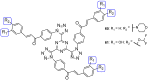
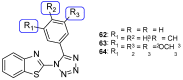
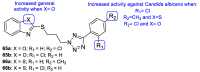
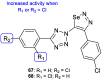
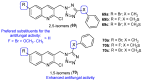
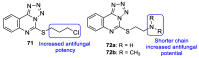
Fungal conditions affect a multitude of people worldwide, leading to increased hospitalization and mortality rates, and the need for novel antifungals is emerging with the rise of resistance and immunocompromised patients. Continuous use of azole drugs, which act by inhibiting the fungal CYP51, involved in the synthesis of ergosterol, essential to the fungal cell membrane, has enhanced the resistance and tolerance of some fungal strains to treatment, thereby limiting the arsenal of available drugs. The goal of this review is to gather literature information on new promising azole developments in clinical trials, with in vitro and in vivo results against fungal strains, and complementary assays, such as toxicity, susceptibility assays, docking studies, among others. Several molecules are reviewed as novel azole structures in clinical trials and with recent/imminent approvals, as well as other innovative molecules with promising antifungal activity. Structure-activity relationship (SAR) studies are displayed whenever possible. The azole moiety is brought over as a privileged structure, with multiple different compounds emerging with distinct pharmacophores and SAR. Particularly, 1,2,3-triazole natural product conjugates emerged in the last years, presenting promising antifungal activity and a broad spectrum against various fungi.
Keywords: antifungal drugs; azoles; new developments.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The Aspergillus fumigatus Damage Resistance Protein Family Coordinately Regulates Ergosterol Biosynthesis and Azole Susceptibility.mBio. 2016 Feb 23;7(1):e01919-15. doi: 10.1128/mBio.01919-15. mBio. 2016. PMID: 26908577 Free PMC article.
-
Oxadiazole-Containing Macrocyclic Peptides Potentiate Azole Activity against Pathogenic Candida Species.mSphere. 2020 Apr 8;5(2):e00256-20. doi: 10.1128/mSphere.00256-20. mSphere. 2020. PMID: 32269162 Free PMC article.
-
High-Throughput Chemical Screen Identifies a 2,5-Disubstituted Pyridine as an Inhibitor of Candida albicans Erg11.mSphere. 2022 Jun 29;7(3):e0007522. doi: 10.1128/msphere.00075-22. Epub 2022 May 9. mSphere. 2022. PMID: 35531664 Free PMC article.
-
New antifungal agents.Dermatol Clin. 2003 Jul;21(3):565-76. doi: 10.1016/s0733-8635(03)00024-x. Dermatol Clin. 2003. PMID: 12956208 Review.
-
Resistance in human pathogenic yeasts and filamentous fungi: prevalence, underlying molecular mechanisms and link to the use of antifungals in humans and the environment.Dan Med J. 2016 Oct;63(10):B5288. Dan Med J. 2016. PMID: 27697142 Review.
Cited by
-
Transcriptomic meta-analysis to identify potential antifungal targets in Candida albicans.BMC Microbiol. 2024 Feb 27;24(1):66. doi: 10.1186/s12866-024-03213-8. BMC Microbiol. 2024. PMID: 38413885 Free PMC article.
-
Anti-Infection of Oral Microorganisms from Herbal Medicine of Piper crocatum Ruiz & Pav.Drug Des Devel Ther. 2024 Jun 25;18:2531-2553. doi: 10.2147/DDDT.S453375. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38952486 Free PMC article. Review.
-
Azole Combinations and Multi-Targeting Drugs That Synergistically Inhibit Candidozyma auris.J Fungi (Basel). 2024 Oct 7;10(10):698. doi: 10.3390/jof10100698. J Fungi (Basel). 2024. PMID: 39452650 Free PMC article. Review.
-
Prophylaxis of Antifungal Drugs against Systemic Fungemia induced by Oral Candidiasis in Mice.Curr Issues Mol Biol. 2023 Feb 4;45(2):1306-1313. doi: 10.3390/cimb45020085. Curr Issues Mol Biol. 2023. PMID: 36826030 Free PMC article.
-
Synthesis, Molecular Docking, and Biological Evaluation of Novel Indole-triazole Conjugates.Curr Drug Discov Technol. 2024;21(6):e120324227917. doi: 10.2174/0115701638295739240222074426. Curr Drug Discov Technol. 2024. PMID: 38482620
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

