The Effects of TRPC6 Knockout in Animal Models of Kidney Disease
- PMID: 36421724
- PMCID: PMC9687984
- DOI: 10.3390/biom12111710
The Effects of TRPC6 Knockout in Animal Models of Kidney Disease
Abstract
Diseases that induce a loss of renal function affect a substantial portion of the world's population and can range from a slight decline in the glomerular filtration rate or microalbuminuria to complete kidney failure. Kidney disorders can be acute or chronic, but any significant reduction in renal function is associated with increased all-cause morbidity and mortality, especially when the conditions become chronic. There is an urgent need for new therapeutic approaches to slow or halt the progression of kidney disease. One potential target of considerable interest is the canonical transient receptor potential-6 (TRPC6) channel. TRCP6 is a cationic channel with a significant permeability to Ca2+. It is expressed in several tissues, including in multiple cell types of the kidney in glomeruli, microvasculature, and tubules. Here, we will describe TRPC6 channels and their roles in signal transduction, with an emphasis on renal cells, and the studies implicating TRPC6 channels in the progression of inherited and acquired kidney diseases. We then describe studies using TRPC6 knockout mice and rats subjected to treatments that model human diseases, including nephrotic syndromes, diabetic nephropathy, autoimmune glomerulonephritis, and acute kidney injuries induced by renal ischemia and by obstruction of the urinary tract. TRPC6 knockout has been shown to reduce glomerular manifestations of disease in several of these models and reduces renal fibrosis caused by urinary tract obstruction. TRPC6 knockout has proven to be less effective at reducing diabetic nephropathy in mouse and rat models. We also summarize the implications of these studies for drug development.
Keywords: TRPC6; diabetic nephropathy; glomerulosclerosis; mesangial cells; podocyte; renal fibrosis.
Conflict of interest statement
Dryer has received speaker’s honoraria from Amgen Inc., Walden Biosciences Inc., and Ardelyx Inc. and currently serves as a scientific advisor to Actio Biosciences Inc. Kim has no conflict to declare.
Figures

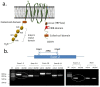
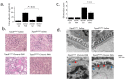
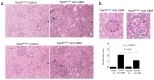
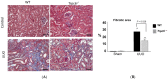
Similar articles
-
TRPC channels: Regulation, dysregulation and contributions to chronic kidney disease.Biochim Biophys Acta Mol Basis Dis. 2019 Jun 1;1865(6):1041-1066. doi: 10.1016/j.bbadis.2019.04.001. Epub 2019 Apr 4. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 30953689 Review.
-
Trpc6 knockout protects against renal fibrosis by restraining the CN‑NFAT2 signaling pathway in T2DM mice.Mol Med Rep. 2024 Jan;29(1):13. doi: 10.3892/mmr.2023.13136. Epub 2023 Dec 1. Mol Med Rep. 2024. PMID: 38038121 Free PMC article.
-
TRPC Channels in Proteinuric Kidney Diseases.Cells. 2019 Dec 23;9(1):44. doi: 10.3390/cells9010044. Cells. 2019. PMID: 31877991 Free PMC article. Review.
-
Role of TRPC6 in Progression of Diabetic Kidney Disease.Curr Hypertens Rep. 2019 May 21;21(7):48. doi: 10.1007/s11906-019-0960-9. Curr Hypertens Rep. 2019. PMID: 31115705 Free PMC article. Review.
-
Trpc6 inactivation confers protection in a model of severe nephrosis in rats.J Mol Med (Berl). 2018 Jul;96(7):631-644. doi: 10.1007/s00109-018-1648-3. Epub 2018 May 22. J Mol Med (Berl). 2018. PMID: 29785489 Free PMC article.
Cited by
-
β-Arrestin pathway activation by selective ATR1 agonism promotes calcium influx in podocytes, leading to glomerular damage.Clin Sci (Lond). 2023 Dec 22;137(24):1789-1804. doi: 10.1042/CS20230313. Clin Sci (Lond). 2023. PMID: 38051199 Free PMC article.
-
Reorganization and Suppression of Store-Operated Calcium Entry in Podocytes of Type 2 Diabetic Rats.Int J Mol Sci. 2023 Apr 14;24(8):7259. doi: 10.3390/ijms24087259. Int J Mol Sci. 2023. PMID: 37108424 Free PMC article.
-
Structural and accessibility studies highlight the differential binding of clemizole to TRPC5 and TRPC6.J Biomol Struct Dyn. 2024 Jan 27:1-14. doi: 10.1080/07391102.2024.2306198. Online ahead of print. J Biomol Struct Dyn. 2024. PMID: 38279926
-
Roles of TRP and PIEZO receptors in autoimmune diseases.Expert Rev Mol Med. 2024 Apr 25;26:e10. doi: 10.1017/erm.2023.23. Expert Rev Mol Med. 2024. PMID: 38659380 Free PMC article. Review.
-
Renal ischaemia-reperfusion injury is promoted by transcription factor NF-kB p65, which inhibits TRPC6 expression by activating miR-150.Clin Hemorheol Microcirc. 2024;86(3):369-382. doi: 10.3233/CH-231979. Clin Hemorheol Microcirc. 2024. PMID: 37980653 Free PMC article.
References
-
- United States Renal Data System . USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD, USA: 2021.
-
- Schaubroeck H., Vandenberghe W., Boer W., Boonen E., Dewulf B., Bourgeois C., Dubois J., Dumoulin A., Fivez T., Gunst J., et al. Acute kidney injury in critical COVID-19: A multicenter cohort analysis in seven large hospitals in Belgium. Crit. Care. 2022;26:225. doi: 10.1186/s13054-022-04086-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

