Elevation of cell-associated HIV-1 transcripts in CSF CD4+ T cells, despite effective antiretroviral therapy, is linked to brain injury
- PMID: 36413502
- PMCID: PMC9860316
- DOI: 10.1073/pnas.2210584119
Elevation of cell-associated HIV-1 transcripts in CSF CD4+ T cells, despite effective antiretroviral therapy, is linked to brain injury
Abstract
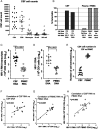
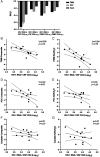
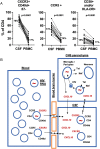
Antiretroviral therapy (ART) can attain prolonged undetectable HIV-1 in plasma and cerebrospinal fluid (CSF), but brain injury remains prevalent in people living with HIV-1 infection (PLHIV). We investigated cell-associated (CA)-HIV-1 RNA transcripts in cells in CSF and blood, using the highly sensitive Double-R assay, together with proton Magnetic Resonance Spectroscopy (1H MRS) of major brain metabolites, in sixteen PLHIV. 14/16 CSF cell samples had quantifiable CA-HIV-1 RNA, at levels significantly higher than in their PBMCs (median 9,266 vs 185 copies /106 CD4+ T-cells; p<0.0001). In individual PLHIV, higher levels of HIV-1 transcripts in CSF cells were associated with greater brain injury in the frontal white matter (Std β=-0.73; p=0.007) and posterior cingulate (Std β=-0.61; p=0.03). 18-colour flow cytometry revealed that the CSF cells were 91% memory T-cells, equally CD4+ and CD8+ T-cells, but fewer B cells (0.4 %), and monocytes (3.1%). CXCR3+CD49d+integrin β7-, CCR5+CD4+ T-cells were highly enriched in CSF, compared with PBMC (p <0.001). However, CA-HIV-1 RNA could not be detected in 10/16 preparations of highly purified monocytes from PBMC, and was extremely low in the other six. Our data show that elevated HIV-1 transcripts in CSF cells were associated with brain injury, despite suppressive ART. The cellular source is most likely memory CD4+ T cells from blood, rather than trafficking monocytes. Future research should focus on inhibitors of this transcription to reduce local production of potentially neurotoxic and inflammatory viral products.
Keywords: CD4+T cells; brain injury; cerebrospinal fluid; intracellular HIV-1 RNA-transcripts; neuropathogenesis.
Conflict of interest statement
The authors declare a competing interest. Kazuo Suzuki is the original inventor under WO2018/045425 (PTC/AU2017/050974) patent, titled “Methods of detecting Lentivirus” of HIV-1 detection targeting “R” region. All other authors report no conflicts of interest.
Figures

Similar articles
-
Comparative Analysis of Cell-Associated HIV DNA Levels in Cerebrospinal Fluid and Peripheral Blood by Droplet Digital PCR.PLoS One. 2015 Oct 2;10(10):e0139510. doi: 10.1371/journal.pone.0139510. eCollection 2015. PLoS One. 2015. PMID: 26431315 Free PMC article.
-
Early Antiretroviral Therapy Is Associated with Lower HIV DNA Molecular Diversity and Lower Inflammation in Cerebrospinal Fluid but Does Not Prevent the Establishment of Compartmentalized HIV DNA Populations.PLoS Pathog. 2017 Jan 3;13(1):e1006112. doi: 10.1371/journal.ppat.1006112. eCollection 2017 Jan. PLoS Pathog. 2017. PMID: 28046096 Free PMC article.
-
Immune Activation and HIV-Specific CD8(+) T Cells in Cerebrospinal Fluid of HIV Controllers and Noncontrollers.AIDS Res Hum Retroviruses. 2016 Aug;32(8):791-800. doi: 10.1089/AID.2015.0313. Epub 2016 May 2. AIDS Res Hum Retroviruses. 2016. PMID: 27019338 Free PMC article.
-
[Deep lung--cellular reaction to HIV].Rev Port Pneumol. 2007 Mar-Apr;13(2):175-212. Rev Port Pneumol. 2007. PMID: 17492233 Review. Portuguese.
-
Cerebrospinal fluid viral escape in HIV patients on antiretroviral therapy: A systematic review of reported cases.Rev Med Virol. 2024 May;34(3):e2536. doi: 10.1002/rmv.2536. Rev Med Virol. 2024. PMID: 38578230 Review.
Cited by
-
Limited HIV-associated neuropathologies and lack of immune activation in sub-saharan African individuals with late-stage subtype C HIV-1 infection.J Neurovirol. 2024 Jun;30(3):303-315. doi: 10.1007/s13365-024-01219-6. Epub 2024 Jun 28. J Neurovirol. 2024. PMID: 38943022
-
Neurosymptomatic HIV-1 CSF escape is associated with replication in CNS T cells and inflammation.J Clin Invest. 2024 Oct 1;134(19):e176358. doi: 10.1172/JCI176358. J Clin Invest. 2024. PMID: 39352388 Free PMC article.
-
IRAK1 inhibition blocks the HIV-1 RNA mediated pro-inflammatory cytokine response from microglia.J Gen Virol. 2023 May;104(5):001858. doi: 10.1099/jgv.0.001858. J Gen Virol. 2023. PMID: 37256770 Free PMC article.
-
HIV transcription persists in the brain of virally suppressed people with HIV.PLoS Pathog. 2024 Aug 8;20(8):e1012446. doi: 10.1371/journal.ppat.1012446. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39116185 Free PMC article.
-
Mechanisms underlying HIV-associated cognitive impairment and emerging therapies for its management.Nat Rev Neurol. 2023 Nov;19(11):668-687. doi: 10.1038/s41582-023-00879-y. Epub 2023 Oct 10. Nat Rev Neurol. 2023. PMID: 37816937 Free PMC article. Review.
References
-
- Mothobi N. Z., Brew B. J., Neurocognitive dysfunction in the highly active antiretroviral therapy era. Curr. Opin. Infect. Dis. 25, 4–9 (2012). - PubMed
-
- Brew B. J., Barnes S. L., The impact of HIV central nervous system persistence on pathogenesis. AIDS 33, S113–S21 (2019). - PubMed
-
- Winston A., Spudich S., Cognitive disorders in people living with HIV. Lancet HIV 7, e504–e513 (2020). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

