Exploratory data on the clinical efficacy of monoclonal antibodies against SARS-CoV-2 Omicron variant of concern
- PMID: 36413383
- PMCID: PMC9681201
- DOI: 10.7554/eLife.79639
Exploratory data on the clinical efficacy of monoclonal antibodies against SARS-CoV-2 Omicron variant of concern
Abstract
Background: Recent in-vitro data have shown that the activity of monoclonal antibodies (mAbs) targeting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) varies according to the variant of concern (VOC). No studies have compared the clinical efficacy of different mAbs against Omicron VOC.
Methods: The MANTICO trial is a non-inferiority randomised controlled trial comparing the clinical efficacy of early treatments with bamlanivimab/etesevimab, casirivimab/imdevimab, and sotrovimab in outpatients aged 50 or older with mild-to-moderate SARS-CoV-2 infection. As the patient enrolment was interrupted for possible futility after the onset of the Omicron wave, the analysis was performed according to the SARS-CoV-2 VOC. The primary outcome was coronavirus disease 2019 (COVID-19) progression (hospitalisation, need of supplemental oxygen therapy, or death through day 14). Secondary outcomes included the time to symptom resolution, assessed using the product-limit method. Kaplan-Meier estimator and Cox proportional hazard model were used to assess the association with predictors. Log rank test was used to compare survival functions.
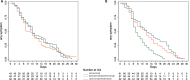
Results: Overall, 319 patients were included. Among 141 patients infected with Delta, no COVID-19 progression was recorded, and the time to symptom resolution did not differ significantly between treatment groups (Log-rank Chi-square 0.22, p 0.90). Among 170 patients infected with Omicron (80.6% BA.1 and 19.4% BA.1.1), two COVID-19 progressions were recorded, both in the bamlanivimab/etesevimab group, and the median time to symptom resolution was 5 days shorter in the sotrovimab group compared with the bamlanivimab/etesevimab and casirivimab/imdevimab groups (HR 0.53 and HR 0.45, 95% CI 0.36-0.77 and 95% CI 0.30-0.67, p<0.01).
Conclusions: Our data suggest that, among adult outpatients with mild-to-moderate SARS-CoV-2 infection due to Omicron BA.1 and BA.1.1, early treatment with sotrovimab reduces the time to recovery compared with casirivimab/imdevimab and bamlanivimab/etesevimab. In the same population, early treatment with casirivimab/imdevimab may maintain a role in preventing COVID-19 progression. The generalisability of trial results is substantially limited by the early discontinuation of the trial and firm conclusions cannot be drawn.
Funding: This trial was funded by the Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA). The VOC identification was funded by the ORCHESTRA (Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic) project, which has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement number 101016167.
Clinical trial number: NCT05205759.
Keywords: Omicron; bamlanivimab/etesevimab; casirivimab/imdevimab; early covid-19; human; medicine; monoclonal antibody; sotrovimab.
© 2022, Mazzaferri et al.
Conflict of interest statement
FM, MM, AS, PD, MM, MT, MA, GD, LC, DD, ES, ED, DG, IM, GS, NM, ET No competing interests declared, LS Lolita Sasset has served as a paid consultant to Abbvie, Janssen, MSD, Gilead Sciences, Janssen, MSD and ViiV Healthcare; she does not have any financial competing interests with this study, AC Annamaria Cattelan has served as a paid consultant to Abbvie, Janssen, MSD, and received research fundings from Gilead Sciences, Janssen, MSD and ViiV Healthcare; she does not have any financial competing interests with this study, CT Carlo Tascini has received grants from Correvio, Biotest, Biomerieux, Gilead, Angelini, MSD, Pfizer, Thermofisher, Zambon, Shionogi, Avir Pharma and Hikma outside the submitted work in the last two years
Figures
Similar articles
-
SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19.Cochrane Database Syst Rev. 2021 Sep 2;9(9):CD013825. doi: 10.1002/14651858.CD013825.pub2. Cochrane Database Syst Rev. 2021. PMID: 34473343 Free PMC article. Review.
-
Clinical efficacy of different monoclonal antibody regimens among non-hospitalised patients with mild to moderate COVID-19 at high risk for disease progression: a prospective cohort study.Eur J Clin Microbiol Infect Dis. 2022 Jul;41(7):1065-1076. doi: 10.1007/s10096-022-04464-x. Epub 2022 Jun 21. Eur J Clin Microbiol Infect Dis. 2022. PMID: 35727429 Free PMC article.
-
Curbing the Delta Surge: Clinical Outcomes After Treatment With Bamlanivimab-Etesevimab, Casirivimab-Imdevimab, or Sotrovimab for Mild to Moderate Coronavirus Disease 2019.Mayo Clin Proc. 2022 Sep;97(9):1641-1648. doi: 10.1016/j.mayocp.2022.06.015. Epub 2022 Jun 23. Mayo Clin Proc. 2022. PMID: 36058578 Free PMC article.
-
Efficacy of Bamlanivimab/Etesevimab and Casirivimab/Imdevimab in Preventing Progression to Severe COVID-19 and Role of Variants of Concern.Infect Dis Ther. 2021 Dec;10(4):2479-2488. doi: 10.1007/s40121-021-00525-4. Epub 2021 Aug 25. Infect Dis Ther. 2021. PMID: 34435337 Free PMC article.
-
SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19.Cochrane Database Syst Rev. 2022 Jun 17;6(6):CD014945. doi: 10.1002/14651858.CD014945.pub2. Cochrane Database Syst Rev. 2022. PMID: 35713300 Free PMC article. Review.
Cited by
-
Sotrovimab therapy elicits antiviral activities against Omicron BQ.1.1 and XBB.1.5 in sera of immunocompromised patients.Med. 2023 Oct 13;4(10):664-667. doi: 10.1016/j.medj.2023.07.007. Med. 2023. PMID: 37837962 Free PMC article.
-
Prescription of Anti-Spike Monoclonal Antibodies in COVID-19 Patients with Resistant SARS-CoV-2 Variants in Italy.Pathogens. 2022 Jul 22;11(8):823. doi: 10.3390/pathogens11080823. Pathogens. 2022. PMID: 35894046 Free PMC article.
-
Within-trial economic analysis of resource use from COMET-ICE: A phase 3 clinical trial evaluating sotrovimab for the treatment of patients with COVID-19 at high risk of progression.J Manag Care Spec Pharm. 2022 Nov;28(11):1261-1271. doi: 10.18553/jmcp.2022.28.11.1261. J Manag Care Spec Pharm. 2022. PMID: 36282931 Free PMC article. Clinical Trial.
-
Efficacy and safety of casirivimab and imdevimab for preventing and treating COVID-19: a systematic review and meta-analysis.J Thorac Dis. 2024 Jun 30;16(6):3606-3622. doi: 10.21037/jtd-23-1604. Epub 2024 Jun 11. J Thorac Dis. 2024. PMID: 38983147 Free PMC article.
-
Current Effective Therapeutics in Management of COVID-19.J Clin Med. 2022 Jul 1;11(13):3838. doi: 10.3390/jcm11133838. J Clin Med. 2022. PMID: 35807123 Free PMC article. Review.
References
-
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell A-M, Simons D, Blomquist PB, Zaidi A, Nash S, Iwani Binti Abdul Aziz N, Thelwall S, Dabrera G, Myers R, Amirthalingam G, Gharbia S, Barrett JC, Elson R, Ladhani SN, Ferguson N, Zambon M, Campbell CNJ, Brown K, Hopkins S, Chand M, Ramsay M, Lopez Bernal J. Covid-19 vaccine effectiveness against the omicron (b.1.1.529) variant. The New England Journal of Medicine. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. - DOI - PMC - PubMed
-
- Arora P, Kempf A, Nehlmeier I, Schulz SR, Cossmann A, Stankov MV, Jäck H-M, Behrens GMN, Pöhlmann S, Hoffmann M. Augmented neutralisation resistance of emerging omicron subvariants BA.2.12.1, BA.4, and BA.5. The Lancet. Infectious Diseases. 2022;22:1117–1118. doi: 10.1016/S1473-3099(22)00422-4. - DOI - PMC - PubMed
-
- Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A, Huang W, Li Q, Wang P, An R, Wang J, Wang Y, Niu X, Yang S, Liang H, Sun H, Li T, Yu Y, Cui Q, Liu S, Yang X, Du S, Zhang Z, Hao X, Shao F, Jin R, Wang X, Xiao J, Wang Y, Xie XS. Omicron escapes the majority of existing SARS-cov-2 neutralizing antibodies. Nature. 2022;602:657–663. doi: 10.1038/s41586-021-04385-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous