Strengthening ethics committees for health-related research in sub-Saharan Africa: a scoping review
- PMID: 36410802
- PMCID: PMC9680187
- DOI: 10.1136/bmjopen-2022-062847
Strengthening ethics committees for health-related research in sub-Saharan Africa: a scoping review
Abstract
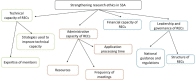
Objective: Health-related research in sub-Saharan Africa (SSA) has grown over the years. However, concerns have been raised about the state of research ethics committees (RECs). This scoping review examines the literature on RECs for health-related research in SSA and identifies strategies that have been applied to strengthen the RECs. It focuses on three aspects of RECs: regulatory governance and leadership, administrative and financial capacity and technical capacity of members.
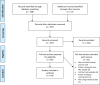
Design: A scoping review of published literature, including grey literature, was conducted using the Joanna Briggs Institute approach.
Data sources: BioOne, CINAHL, Embase (via Ovid), Education Abstracts, Global Health, Google Scholar, Jstor, OpenEdition (French), Philosopher's Index, PsycINFO, PubMed, Science Citation and Expanded Index (Web of Science), reference lists of included studies and specific grey literature sources.
Eligibility criteria: We included empirical studies on RECs for health-related research in SSA, covering topics on REC leadership and governance, administrative and financial capacity and the technical capacity of REC members. We included studies published between 01 January 2000 and 18 February 2022 and written in English, French, Portuguese or Swahili.
Data extraction and synthesis: Two independent reviewers screened the records. Data were extracted by one reviewer and cross-checked by another. Owing to the heterogeneity of included studies, thematic analysis was used.
Results: We included 54 studies. The findings show that most RECs in SSA work under significant administrative and financial constraints, with few opportunities for capacity building for committee members. This has an impact on the quality of reviews and the overall performance of RECs. Although most countries have national governance systems for RECs, they lack regulations on accountability, transparency and monitoring of RECs.
Conclusions: This review provides a comprehensive overview of the literature on RECs for health-related research in SSA and contributes to our understanding of how RECs can be strengthened.
Keywords: Health & safety; International health services; MEDICAL ETHICS.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Erratum: Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs.J Vis Exp. 2023 May 26;(195). doi: 10.3791/6561. J Vis Exp. 2023. PMID: 37235796
-
Ethics of Procuring and Using Organs or Tissue from Infants and Newborns for Transplantation, Research, or Commercial Purposes: Protocol for a Bioethics Scoping Review.Wellcome Open Res. 2024 Dec 5;9:717. doi: 10.12688/wellcomeopenres.23235.1. eCollection 2024. Wellcome Open Res. 2024. PMID: 39839977 Free PMC article.
-
Unlocking data: Decision-maker perspectives on cross-sectoral data sharing and linkage as part of a whole-systems approach to public health policy and practice.Public Health Res (Southampt). 2024 Nov 20:1-30. doi: 10.3310/KYTW2173. Online ahead of print. Public Health Res (Southampt). 2024. PMID: 39582242
-
Exploring conceptual and theoretical frameworks for nurse practitioner education: a scoping review protocol.JBI Database System Rev Implement Rep. 2015 Oct;13(10):146-55. doi: 10.11124/jbisrir-2015-2150. JBI Database System Rev Implement Rep. 2015. PMID: 26571290
-
The use of Open Dialogue in Trauma Informed Care services for mental health consumers and their family networks: A scoping review.J Psychiatr Ment Health Nurs. 2024 Aug;31(4):681-698. doi: 10.1111/jpm.13023. Epub 2024 Jan 17. J Psychiatr Ment Health Nurs. 2024. PMID: 38230967
Cited by
-
The promise of data science for health research in Africa.Nat Commun. 2023 Sep 29;14(1):6084. doi: 10.1038/s41467-023-41809-2. Nat Commun. 2023. PMID: 37770478 Free PMC article. Review.
-
Health Data Sciences and Cardiovascular Disease in Africa: Needs and the Way Forward.Curr Atheroscler Rep. 2024 Nov;26(11):659-671. doi: 10.1007/s11883-024-01235-1. Epub 2024 Sep 6. Curr Atheroscler Rep. 2024. PMID: 39240493 Review.
-
Behind the scenes of research ethics committee oversight: a qualitative research study with committee chairs in the Middle East and North Africa region.BMC Med Ethics. 2024 Aug 8;25(1):86. doi: 10.1186/s12910-024-01083-3. BMC Med Ethics. 2024. PMID: 39118102 Free PMC article.
-
Understanding the language barriers to translating informed consent documents for maternal health trials in Zambia: a qualitative study.BMJ Open. 2024 Apr 5;14(4):e076744. doi: 10.1136/bmjopen-2023-076744. BMJ Open. 2024. PMID: 38580359 Free PMC article.
References
-
- Bryant JH, Harrison PF. Health research: essential link to equity in development, in Global Health in Transition: A Synthesis: Perspectives from International Organizations. National Academies Press (US), 1996. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials