Combined monitoring of IgG and IgA anti-Spike and anti-Receptor binding domain long term responses following BNT162b2 mRNA vaccination in Greek healthcare workers
- PMID: 36409702
- PMCID: PMC9678302
- DOI: 10.1371/journal.pone.0277827
Combined monitoring of IgG and IgA anti-Spike and anti-Receptor binding domain long term responses following BNT162b2 mRNA vaccination in Greek healthcare workers
Abstract
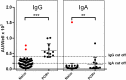
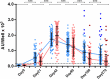
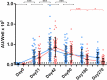
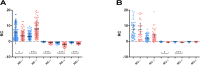
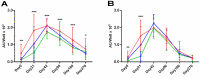
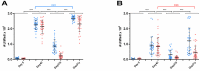
Studies on the humoral response to homologous BNT162b2 mRNA-vaccination focus mainly on IgG antibody dynamics, while long-term IgA kinetics are understudied. Herein, kinetics of IgG and IgA levels against trimeric-Spike (S) and Receptor-Binding-Domain (RBD) were evaluated by in-house ELISAs in 146 two-dose vaccinated Greek healthcare workers (HCWs) in a 9-month period at six time points (up to 270 days after the first dose). The effect of a homologous booster third dose was also studied and evaluated. The peak of immune response was observed 21 days after the second dose; 100% seroconversion rate for anti-S and anti-RBD IgG, and 99.7% and 96.3% respectively for IgA. IgG antibody levels displayed higher increase compared to IgA. Declining but persistent anti-SARS-CoV-2 antibody levels were detected 9 months after vaccination; IgG and IgA anti-S levels approached those after the first dose, while a more rapid reduction rate for anti-RBD antibodies led to significantly lower levels for both classes, supporting the need for a booster dose. Indeed, a homologous booster third dose resulted in enhanced levels of anti-S of both classes, whereas anti-RBD didn't exceed the peak levels after the second dose. Previous SARS-CoV-2 infection, flu vaccination, BMI<35 and the occurrence of an adverse event upon vaccination, were associated with higher IgG antibody levels over time, which however were negatively affected by age increase and the presence of chronic diseases. Overall, after concurrently using the S and RBD target-antigens in in-house ELISAs, we report in addition to IgG, long-term persistence of IgA antibodies. Regarding antibody levels, homologous mRNA vaccination gives rise to an effective anti-viral protection up to 9 months negatively correlated to age. Considering that COVID-19 is still a matter of public concern, booster vaccine doses remain critical to vulnerable individuals.
Copyright: © 2022 Sarrigeorgiou et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Durability of anti-spike antibodies after vaccination with mRNA SARS-CoV-2 vaccine is longer in subjects with previous infection: could the booster dose be delayed?Infection. 2022 Dec;50(6):1573-1577. doi: 10.1007/s15010-022-01816-9. Epub 2022 Apr 7. Infection. 2022. PMID: 35391649 Free PMC article.
-
Kinetics and Persistence of the Cellular and Humoral Immune Responses to BNT162b2 mRNA Vaccine in SARS-CoV-2-Naive and -Experienced Subjects: Impact of Booster Dose and Breakthrough Infections.Front Immunol. 2022 May 31;13:863554. doi: 10.3389/fimmu.2022.863554. eCollection 2022. Front Immunol. 2022. PMID: 35711445 Free PMC article.
-
Dynamic of anti-spike receptor binding domain (RBD) levels and short-term adverse events following a heterologous booster dose of BNT162b2 after two doses of CoronaVac in Thai health care workers.Vaccine. 2022 May 9;40(21):2915-2924. doi: 10.1016/j.vaccine.2022.04.020. Epub 2022 Apr 13. Vaccine. 2022. PMID: 35430106 Free PMC article.
-
Follow-Up and Comparative Assessment of SARS-CoV-2 IgA, IgG, Neutralizing, and Total Antibody Responses After BNT162b2 or mRNA-1273 Heterologous Booster Vaccination.Influenza Other Respir Viruses. 2024 May;18(5):e13290. doi: 10.1111/irv.13290. Influenza Other Respir Viruses. 2024. PMID: 38706402 Free PMC article.
Cited by
-
Novel Competitive ELISA Utilizing Trimeric Spike Protein of SARS-CoV-2, Could Identify More Than RBD-RBM Specific Neutralizing Antibodies in Hybrid Sera.Vaccines (Basel). 2024 Aug 13;12(8):914. doi: 10.3390/vaccines12080914. Vaccines (Basel). 2024. PMID: 39204038 Free PMC article.
-
Seroepidemiological assessment of SARS-CoV-2 vaccine responsiveness and associated factors in the vaccinated community of the Casablanca-Settat Region, Morocco.Sci Rep. 2024 Apr 3;14(1):7817. doi: 10.1038/s41598-024-58498-6. Sci Rep. 2024. PMID: 38570577 Free PMC article.
-
Long-Term Immunological Alertness and Response to COVID-19 Vaccination-Conditions for Prevention in Early Palliative Oncological Care Patients.Vaccines (Basel). 2024 Mar 13;12(3):299. doi: 10.3390/vaccines12030299. Vaccines (Basel). 2024. PMID: 38543933 Free PMC article.
-
A Longitudinal Study in Tunisia to Assess the Anti-RBD IgG and IgA Responses Induced by Three Different COVID-19 Vaccine Platforms.Trop Med Infect Dis. 2024 Mar 13;9(3):61. doi: 10.3390/tropicalmed9030061. Trop Med Infect Dis. 2024. PMID: 38535885 Free PMC article.
-
Humoral immunity against SARS-CoV-2 evoked by heterologous vaccination groups using the CoronaVac (Sinovac) and BNT162b2 (Pfizer/BioNTech) vaccines in Chile.Front Public Health. 2023 Aug 24;11:1229045. doi: 10.3389/fpubh.2023.1229045. eCollection 2023. Front Public Health. 2023. PMID: 37693706 Free PMC article.
References
-
- Comirnaty and Pfizer-BioNTech COVID-19 Vaccine | FDA. [cited 18 Apr 2022]. Available: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

