Efficacy and Safety of Sofosbuvir-based Regimens in Hepatitis C Patients With Decompensated Cirrhosis: A Systematic Review and Meta-analysis
- PMID: 36406321
- PMCID: PMC9647115
- DOI: 10.14218/JCTH.2022.00006
Efficacy and Safety of Sofosbuvir-based Regimens in Hepatitis C Patients With Decompensated Cirrhosis: A Systematic Review and Meta-analysis
Abstract
Background and aims: Decompensated cirrhotic patients with hepatitis C (HCV) are often under-represented in clinical trials. We aimed to evaluate pooled data on the efficacy and safety of sofosbuvir (SOF)-based regimens in these patients.
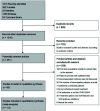
Methods: We conducted a systemic review and meta-analysis by searching multiple databases for studies published from October 2010 to October 2020. Outcomes of interest were sustained virologic response (SVR) and safety of SOF-based regimens in decompensated HCV patients. Two reviewers independently performed the study selection and data extraction.
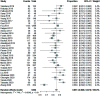
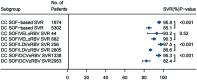
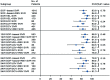
Results: We included 33 studies that enrolled 5,302 HCV patients. The pooled SVR rate in decompensated patients with SOF-based regimens was 85.1% (95% CI: 82.8-87.3). Patients on SOF/velpatasvir±ribavirin achieved a significantly higher SVR (91.0%, 95% CI: 87.7-93.9) than that of SOF/ledipasvir±ribavirin [(86.3%, 95% CI: 84.6-87.8); p=0.004)], or on SOF/daclatasvir±ribavirin (82.4%, 95% CI: 78.2-86.2%; p<0.001). Adding ribavirin to SOF-based regimens (pooled SVR 84.9%, 95% CI: 81.7-87.9) did not significantly increase the SVR [(83.8% (95% CI: 76.8-89.8%; p=0.76)] in decompensated patients, which was also true in subgroup analyses for each regimen within the same treatment duration. However, adding ribavirin significantly increased the frequency of adverse events from 52.9% (95% CI: 28.0-77.1) to 89.2% (95% CI: 68.1-99.9) and frequency of severe events. The pooled incidence of hepatocellular carcinoma and case-fatality of decompensated patients were 3.1% (95% CI: 1.5-5.0) and 4.6% (95% CI: 3.1-6.3), respectively. The overall heterogeneity was high. There was no publication bias.
Conclusions: The analysis found that 12 weeks of SOF/velpatasvir without ribavirin is the preferred therapy, with a significantly higher SVR compared with other SOF-based regimens in decompensated HCV patients.
Keywords: Direct-acting antiviral; HCV liver failure; Ribavirin; Sustained virologic response.
© 2023 Authors.
Conflict of interest statement
CQP is a speaker and consultant for Gilead Sciences. He also received a research grant from Gilead Sciences and Assembly Biosciences. The other authors have no conflict of interests related to this publication.
Figures




Similar articles
-
Results of Sofosbuvir Plus Ribavirin in Patients With Hepatitis C Related Decompensated Cirrhosis.J Clin Exp Hepatol. 2019 Jan-Feb;9(1):4-12. doi: 10.1016/j.jceh.2018.02.009. Epub 2018 Mar 6. J Clin Exp Hepatol. 2019. PMID: 30765933 Free PMC article.
-
Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3.J Hepatol. 2019 Jan;70(1):15-23. doi: 10.1016/j.jhep.2018.09.018. Epub 2018 Sep 26. J Hepatol. 2019. PMID: 30266283
-
Adjuvant ribavirin and longer direct-acting antiviral treatment duration improve sustained virological response among hepatitis C patients at risk of treatment failure.J Viral Hepat. 2019 Oct;26(10):1210-1217. doi: 10.1111/jvh.13162. Epub 2019 Jul 4. J Viral Hepat. 2019. PMID: 31197910 Free PMC article.
-
Efficacy and safety outcomes of sofosbuvir-based treatment regimens for hepatitis C virus-infected patients with or without cirrhosis from phase III clinical trials.Ther Clin Risk Manag. 2017 Apr 12;13:477-497. doi: 10.2147/TCRM.S134818. eCollection 2017. Ther Clin Risk Manag. 2017. PMID: 28442915 Free PMC article. Review.
-
Safety and efficacy of sofosbuvir plus ledipasvir with and without ribavirin for chronic HCV genotype-1 infection: a systematic review and meta-analysis.Antivir Ther. 2017;22(5):369-379. doi: 10.3851/IMP3083. Epub 2016 Sep 2. Antivir Ther. 2017. PMID: 27588749 Review.
Cited by
-
Efficacy and Safety of Sofosbuvir/Velpatasvir Plus Ribavirin in Patients with Hepatitis C Virus-Related Decompensated Cirrhosis.Viruses. 2023 Sep 29;15(10):2026. doi: 10.3390/v15102026. Viruses. 2023. PMID: 37896803 Free PMC article.
-
Outcomes of direct-acting antivirals in patients with HCV decompensated cirrhosis: a systematic review and meta-analysis.Front Med (Lausanne). 2023 Nov 29;10:1295857. doi: 10.3389/fmed.2023.1295857. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38093978 Free PMC article.
References
LinkOut - more resources
Full Text Sources
