Ferroptosis and its role in skeletal muscle diseases
- PMID: 36406272
- PMCID: PMC9669482
- DOI: 10.3389/fmolb.2022.1051866
Ferroptosis and its role in skeletal muscle diseases
Abstract
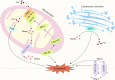
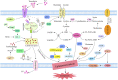
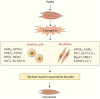
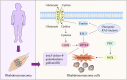
Ferroptosis is characterized by the accumulation of iron and lipid peroxidation products, which regulates physiological and pathological processes in numerous organs and tissues. A growing body of research suggests that ferroptosis is a key causative factor in a variety of skeletal muscle diseases, including sarcopenia, rhabdomyolysis, rhabdomyosarcoma, and exhaustive exercise-induced fatigue. However, the relationship between ferroptosis and various skeletal muscle diseases has not been investigated systematically. This review's objective is to provide a comprehensive summary of the mechanisms and signaling factors that regulate ferroptosis, including lipid peroxidation, iron/heme, amino acid metabolism, and autophagy. In addition, we tease out the role of ferroptosis in the progression of different skeletal muscle diseases and ferroptosis as a potential target for the treatment of multiple skeletal muscle diseases. This review can provide valuable reference for the research on the pathogenesis of skeletal muscle diseases, as well as for clinical prevention and treatment.
Keywords: fatigue; ferroptosis; mechanism; myositis; rhabdomyolysis; rhabdomyosarcoma; sarcopenia.
Copyright © 2022 Wang, Zhang, Jiao, Wang, Wang, Zhao, Fan, Tian, Li and Mi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Ferroptosis in a sarcopenia model of senescence accelerated mouse prone 8 (SAMP8).Int J Biol Sci. 2021 Jan 1;17(1):151-162. doi: 10.7150/ijbs.53126. eCollection 2021. Int J Biol Sci. 2021. PMID: 33390840 Free PMC article.
-
Emerging Potential Therapeutic Targets of Ferroptosis in Skeletal Diseases.Oxid Med Cell Longev. 2022 Jul 30;2022:3112388. doi: 10.1155/2022/3112388. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35941905 Free PMC article. Review.
-
ACSL4 contributes to ferroptosis-mediated rhabdomyolysis in exertional heat stroke.J Cachexia Sarcopenia Muscle. 2022 Jun;13(3):1717-1730. doi: 10.1002/jcsm.12953. Epub 2022 Mar 3. J Cachexia Sarcopenia Muscle. 2022. PMID: 35243801 Free PMC article.
-
Ferroptosis triggers airway inflammation in asthma.Ther Adv Respir Dis. 2023 Jan-Dec;17:17534666231208628. doi: 10.1177/17534666231208628. Ther Adv Respir Dis. 2023. PMID: 37947059 Free PMC article. Review.
-
Ferroptosis and musculoskeletal diseases: "Iron Maiden" cell death may be a promising therapeutic target.Front Immunol. 2022 Oct 11;13:972753. doi: 10.3389/fimmu.2022.972753. eCollection 2022. Front Immunol. 2022. PMID: 36304454 Free PMC article. Review.
Cited by
-
ABCG2 Gene Expression in Non-Small Cell Lung Cancer.Biomedicines. 2024 Oct 19;12(10):2394. doi: 10.3390/biomedicines12102394. Biomedicines. 2024. PMID: 39457707 Free PMC article.
-
Class IIa HDACs inhibit cell death pathways and protect muscle integrity in response to lipotoxicity.Cell Death Dis. 2023 Dec 1;14(12):787. doi: 10.1038/s41419-023-06319-5. Cell Death Dis. 2023. PMID: 38040704 Free PMC article.
-
Identification of ferroptosis-associated genes and potential pharmacological targets in sepsis-induced myopathy.Heliyon. 2024 Mar 30;10(7):e29062. doi: 10.1016/j.heliyon.2024.e29062. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38601693 Free PMC article.
-
Iron status and sarcopenia-related traits: a bi-directional Mendelian randomization study.Sci Rep. 2024 Apr 22;14(1):9179. doi: 10.1038/s41598-024-60059-w. Sci Rep. 2024. PMID: 38649459 Free PMC article.
-
Expression of Prostaglandin Genes and β-Catenin in Whole Blood as Potential Markers of Muscle Degeneration.Int J Mol Sci. 2023 Aug 17;24(16):12885. doi: 10.3390/ijms241612885. Int J Mol Sci. 2023. PMID: 37629065 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

