Bacterial polyphosphates induce CXCL4 and synergize with complement anaphylatoxin C5a in lung injury
- PMID: 36405694
- PMCID: PMC9669059
- DOI: 10.3389/fimmu.2022.980733
Bacterial polyphosphates induce CXCL4 and synergize with complement anaphylatoxin C5a in lung injury
Abstract
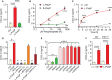
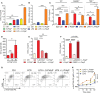
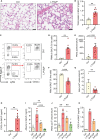
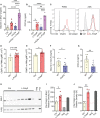
Polyphosphates are linear polymers of inorganic phosphates that exist in all living cells and serve pleiotropic functions. Bacteria produce long-chain polyphosphates, which can interfere with host defense to infection. In contrast, short-chain polyphosphates are released from platelet dense granules and bind to the chemokine CXCL4. Here, we report that long-chain polyphosphates induced the release of CXCL4 from mouse bone marrow-derived macrophages and peritoneal macrophages in a dose-/time-dependent fashion resulting from an induction of CXCL4 mRNA. This polyphosphate effect was lost after pre-incubation with recombinant exopolyphosphatase (PPX) Fc fusion protein, demonstrating the potency of long chains over monophosphates and ambient cations. In detail, polyphosphate chains >70 inorganic phosphate residues were required to reliably induce CXCL4. Polyphosphates acted independently of the purinergic P2Y1 receptor and the MyD88/TRIF adaptors of Toll-like receptors. On the other hand, polyphosphates augmented LPS/MyD88-induced CXCL4 release, which was explained by intracellular signaling convergence on PI3K/Akt. Polyphosphates induced Akt phosphorylation at threonine-308. Pharmacologic blockade of PI3K (wortmannin, LY294002) antagonized polyphosphate-induced CXCL4 release from macrophages. Intratracheal polyphosphate administration to C57BL/6J mice caused histologic signs of lung injury, disruption of the endothelial-epithelial barrier, influx of Ly6G+ polymorphonuclear neutrophils, depletion of CD11c+SiglecF+ alveolar macrophages, and release of CXCL4. Long-chain polyphosphates synergized with the complement anaphylatoxin, C5a, which was partly explained by upregulation of C5aR1 on myeloid cells. C5aR1-/- mice were protected from polyphosphate-induced lung injury. C5a generation occurred in the lungs and bronchoalveolar lavage fluid (BALF) of polyphosphate-treated C57BL/6J mice. In conclusion, we demonstrate that polyphosphates govern immunomodulation in macrophages and promote acute lung injury.
Keywords: acute respiratory distress syndrome; immunology; infection; innate immunity; platelet factor 4; sepsis.
Copyright © 2022 Roewe, Walachowski, Sharma, Berthiaume, Reinhardt and Bosmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Immunomodulation of neutrophil granulocyte functions by bacterial polyphosphates.Eur J Immunol. 2023 May;53(5):e2250339. doi: 10.1002/eji.202250339. Epub 2023 Apr 24. Eur J Immunol. 2023. PMID: 36959687 Free PMC article.
-
Experimental design of complement component 5a-induced acute lung injury (C5a-ALI): a role of CC-chemokine receptor type 5 during immune activation by anaphylatoxin.FASEB J. 2015 Sep;29(9):3762-72. doi: 10.1096/fj.15-271635. Epub 2015 May 21. FASEB J. 2015. PMID: 25999468 Free PMC article.
-
MyD88-dependent production of IL-17F is modulated by the anaphylatoxin C5a via the Akt signaling pathway.FASEB J. 2011 Dec;25(12):4222-32. doi: 10.1096/fj.11-191205. Epub 2011 Aug 22. FASEB J. 2011. PMID: 21859896 Free PMC article.
-
The harmful role of c5a on innate immunity in sepsis.J Innate Immun. 2010;2(5):439-45. doi: 10.1159/000317194. Epub 2010 Jun 26. J Innate Immun. 2010. PMID: 20588003 Free PMC article. Review.
-
Complement as a Major Inducer of Harmful Events in Infectious Sepsis.Shock. 2020 Nov;54(5):595-605. doi: 10.1097/SHK.0000000000001531. Shock. 2020. PMID: 32187106 Free PMC article. Review.
Cited by
-
Immunomodulation of neutrophil granulocyte functions by bacterial polyphosphates.Eur J Immunol. 2023 May;53(5):e2250339. doi: 10.1002/eji.202250339. Epub 2023 Apr 24. Eur J Immunol. 2023. PMID: 36959687 Free PMC article.
-
Immunity of turbot Induced by inactivated vaccine of Aeromonas salmonicida from the perspective of DNA methylation.Front Immunol. 2023 Feb 7;14:1124322. doi: 10.3389/fimmu.2023.1124322. eCollection 2023. Front Immunol. 2023. PMID: 36845093 Free PMC article.
-
Aging is associated with an insufficient early inflammatory response of lung endothelial cells in SARS-CoV-2 infection.Front Immunol. 2024 Jun 7;15:1397990. doi: 10.3389/fimmu.2024.1397990. eCollection 2024. Front Immunol. 2024. PMID: 38911865 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

