Recent progress of gene circuit designs in immune cell therapies
- PMID: 36395726
- PMCID: PMC9681026
- DOI: 10.1016/j.cels.2022.09.006
Recent progress of gene circuit designs in immune cell therapies
Abstract
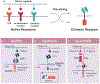
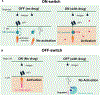
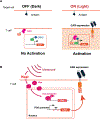
The success of chimeric antigen receptor (CAR) T cell therapy against hematological cancers has convincingly demonstrated the potential of using genetically engineered cells as therapeutic agents. Although much progress has been achieved in cell therapy, more beneficial capabilities have yet to be fully explored. One of the unique advantages afforded by cell therapies is the possibility to implement genetic control circuits, which enables diverse signal sensing and logical processing for optimal response in the complex tumor microenvironment. In this perspective, we will first outline design considerations for cell therapy control circuits that address clinical demands. We will compare and contrast key design features in some of the latest control circuits developments and conclude by discussing potential future directions.
Keywords: CAR; gene circuits; immune cell therapy; synthetic biology.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests W.W.W. is a co-founder and shareholder of Senti Biosciences. A.S.K. is a shareholder of Senti Bioscience and Chroma Medicine. S.L. is a current employee of Sanofi.
Figures


Similar articles
-
Chimeric Antigen Receptor T-Cell Therapy: Current Perspective on T Cell-Intrinsic, T Cell-Extrinsic, and Therapeutic Limitations.Cancer J. 2023 Jan-Feb 01;29(1):28-33. doi: 10.1097/PPO.0000000000000636. Cancer J. 2023. PMID: 36693155 Review.
-
Recent findings on chimeric antigen receptor (CAR)-engineered immune cell therapy in solid tumors and hematological malignancies.Stem Cell Res Ther. 2022 Sep 24;13(1):482. doi: 10.1186/s13287-022-03163-w. Stem Cell Res Ther. 2022. PMID: 36153626 Free PMC article. Review.
-
Challenges and Prospects of Chimeric Antigen Receptor T-cell Therapy for Metastatic Prostate Cancer.Eur Urol. 2020 Mar;77(3):299-308. doi: 10.1016/j.eururo.2019.08.014. Epub 2019 Aug 28. Eur Urol. 2020. PMID: 31471138 Review.
-
Accelerating the development of genetically engineered cellular therapies: a framework for extrapolating data across related products.Cytotherapy. 2024 Jul;26(7):778-784. doi: 10.1016/j.jcyt.2024.03.009. Epub 2024 Mar 16. Cytotherapy. 2024. PMID: 38583170
-
Biomaterials for chimeric antigen receptor T cell engineering.Acta Biomater. 2023 Aug;166:1-13. doi: 10.1016/j.actbio.2023.04.043. Epub 2023 May 2. Acta Biomater. 2023. PMID: 37137403 Review.
Cited by
-
Orthogonal inducible control of Cas13 circuits enables programmable RNA regulation in mammalian cells.Nat Commun. 2024 Feb 21;15(1):1572. doi: 10.1038/s41467-024-45795-x. Nat Commun. 2024. PMID: 38383558 Free PMC article.
-
Mechanical forces amplify TCR mechanotransduction in T cell activation and function.Appl Phys Rev. 2024 Mar;11(1):011304. doi: 10.1063/5.0166848. Appl Phys Rev. 2024. PMID: 38434676 Review.
-
High-throughput functional characterization of combinations of transcriptional activators and repressors.Cell Syst. 2023 Sep 20;14(9):746-763.e5. doi: 10.1016/j.cels.2023.07.001. Epub 2023 Aug 4. Cell Syst. 2023. PMID: 37543039 Free PMC article.
-
Arming CAR-T cells with cytokines and more: Innovations in the fourth-generation CAR-T development.Mol Ther. 2023 Nov 1;31(11):3146-3162. doi: 10.1016/j.ymthe.2023.09.021. Epub 2023 Oct 5. Mol Ther. 2023. PMID: 37803832 Free PMC article. Review.
-
Programmable synthetic receptors: the next-generation of cell and gene therapies.Signal Transduct Target Ther. 2024 Jan 3;9(1):7. doi: 10.1038/s41392-023-01680-5. Signal Transduct Target Ther. 2024. PMID: 38167329 Free PMC article. Review.
References
-
- FDA, U.S. (2021). BREYANZI (lisocabtagene maraleucel).
-
- FDA, U.S. (2021). ABECMA (idecabtagene vicleucel).
-
- FDA, U.S. (2017). KYMRIAH (tisagenlecleucel). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

