Afucosylation of HLA-specific IgG1 as a potential predictor of antibody pathogenicity in kidney transplantation
- PMID: 36384101
- PMCID: PMC9729883
- DOI: 10.1016/j.xcrm.2022.100818
Afucosylation of HLA-specific IgG1 as a potential predictor of antibody pathogenicity in kidney transplantation
Abstract
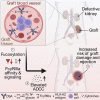
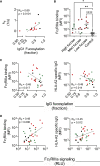
Antibody-mediated rejection (AMR) is the leading cause of graft failure. While donor-specific antibodies (DSAs) are associated with a higher risk of AMR, not all patients with DSAs develop rejection, suggesting that the characteristics of alloantibodies determining their pathogenicity remain undefined. Using human leukocyte antigen (HLA)-A2-specific antibodies as a model, we apply systems serology tools to investigate qualitative features of immunoglobulin G (IgG) alloantibodies including Fc-glycosylation patterns and FcγR-binding properties. Levels of afucosylated anti-A2 antibodies are elevated in seropositive patients, especially those with AMR, suggesting potential cytotoxicity via FcγRIII-mediated mechanisms. Afucosylation of both glycoengineered monoclonal and naturally glycovariant polyclonal serum IgG specific to HLA-A2 drives potentiated binding to, slower dissociation from, and enhanced signaling through FcγRIII, a receptor widely expressed on innate effector cells, and greater cytotoxicity against HLA-A2+ cells mediated by natural killer (NK) cells. Collectively, these results suggest that afucosylated DSA may be a biomarker of AMR and contribute to pathogenesis.
Keywords: ADCC; IgG; afucosylation; antibody-mediated rejection; donor-specific antibody; effector function; glycosylation; transplantation.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests P.B., S.S., T.P., A.L.M., N.d.H., M.W., A.M., and M.E.A. are named inventors on a provisional patent application related to this work.
Figures
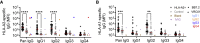



Comment in
-
Sugar and spice: Fc glycosylation in antibody-mediated transplant rejection.Cell Rep Med. 2022 Nov 15;3(11):100809. doi: 10.1016/j.xcrm.2022.100809. Cell Rep Med. 2022. PMID: 36384088 Free PMC article.
Similar articles
-
Characteristics of Circulating Donor Human Leukocyte Antigen-specific Immunoglobulin G Antibodies Predictive of Acute Antibody-mediated Rejection and Kidney Allograft Failure.Transplantation. 2015 Jun;99(6):1156-64. doi: 10.1097/TP.0000000000000511. Transplantation. 2015. PMID: 25629531 Free PMC article.
-
Importance of human leukocyte antigen antibodies and leukocyte antigen/killer-cell immunoglobulin-like receptor genes in liver transplantation.World J Gastroenterol. 2023 Feb 7;29(5):766-772. doi: 10.3748/wjg.v29.i5.766. World J Gastroenterol. 2023. PMID: 36816626 Free PMC article.
-
Determining donor-specific antibody C1q-binding ability improves the prediction of antibody-mediated rejection in human leucocyte antigen-incompatible kidney transplantation.Transpl Int. 2017 Apr;30(4):347-359. doi: 10.1111/tri.12873. Epub 2016 Nov 2. Transpl Int. 2017. PMID: 27717025
-
Effect of C1q-binding donor-specific anti-HLA antibodies on the clinical outcomes of patients after renal transplantation: A systematic review and meta-analysis.Transpl Immunol. 2022 Jun;72:101566. doi: 10.1016/j.trim.2022.101566. Epub 2022 Mar 4. Transpl Immunol. 2022. PMID: 35257893 Review.
-
Donor-Specific Antibodies in Kidney Transplant Recipients.Clin J Am Soc Nephrol. 2018 Jan 6;13(1):182-192. doi: 10.2215/CJN.00700117. Epub 2017 Apr 26. Clin J Am Soc Nephrol. 2018. PMID: 28446536 Free PMC article. Review.
Cited by
-
Antibody-mediated rejection: prevention, monitoring and treatment dilemmas.Curr Opin Organ Transplant. 2022 Oct 1;27(5):405-414. doi: 10.1097/MOT.0000000000001011. Epub 2022 Aug 11. Curr Opin Organ Transplant. 2022. PMID: 35950887 Free PMC article. Review.
-
Cellular surface plasmon resonance-based detection of anti-HPA-1a antibody glycosylation in fetal and neonatal alloimmune thrombocytopenia.Front Immunol. 2023 Oct 5;14:1225603. doi: 10.3389/fimmu.2023.1225603. eCollection 2023. Front Immunol. 2023. PMID: 37868955 Free PMC article.
-
Sugar and spice: Fc glycosylation in antibody-mediated transplant rejection.Cell Rep Med. 2022 Nov 15;3(11):100809. doi: 10.1016/j.xcrm.2022.100809. Cell Rep Med. 2022. PMID: 36384088 Free PMC article.
-
The Role of Clinical Glyco(proteo)mics in Precision Medicine.Mol Cell Proteomics. 2023 Jun;22(6):100565. doi: 10.1016/j.mcpro.2023.100565. Epub 2023 May 9. Mol Cell Proteomics. 2023. PMID: 37169080 Free PMC article.
-
New insights into maladaptive vascular responses to donor specific HLA antibodies in organ transplantation.Front Transplant. 2023 Apr 28;2:1146040. doi: 10.3389/frtra.2023.1146040. eCollection 2023. Front Transplant. 2023. PMID: 38993843 Free PMC article. Review.
References
-
- Cheng E.Y., Everly M.J., Kaneku H., Banuelos N., Wozniak L.J., Venick R.S., Marcus E.A., McDiarmid S.V., Busuttil R.W., Terasaki P.I., Farmer D.G. Prevalence and clinical impact of donor-specific alloantibody among intestinal transplant recipients. Transplantation. 2017;101:873–882. doi: 10.1097/tp.0000000000001391. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

