Mesenchymal Stem Cells Attenuate Asthmatic Inflammation and Airway Remodeling by Modulating Macrophages/Monocytes in the IL-13-Overexpressing Mouse Model
- PMID: 36381962
- PMCID: PMC9634145
- DOI: 10.4110/in.2022.22.e40
Mesenchymal Stem Cells Attenuate Asthmatic Inflammation and Airway Remodeling by Modulating Macrophages/Monocytes in the IL-13-Overexpressing Mouse Model
Abstract
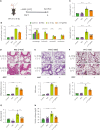
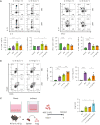
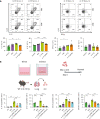
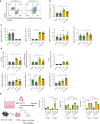
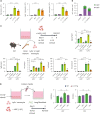
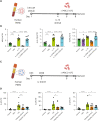
Mesenchymal stem cells (MSCs) are attractive alternatives to conventional anti-asthmatic drugs for severe asthma. Mechanisms underlying the anti-asthmatic effects of MSCs have not yet been elucidated. This study evaluated the anti-asthmatic effects of intravenously administered MSCs, focusing on macrophages and monocytes. Seven-week-old transgenic (Tg) mice with lung-specific overexpression of IL-13 were used to simulate chronic asthma. MSCs were intravenously administered four days before sampling. We examined changes in immune cell subpopulations, gene expression, and histological phenotypes. IL-13 Tg mice exhibited diverse features of chronic asthma, including severe type 2 inflammation, airway fibrosis, and mucus metaplasia. Intravenous administration of MSCs attenuated these asthmatic features just four days after a single treatment. MSC treatment significantly reduced SiglecF-CD11c-CD11b+ monocyte-derived macrophages (MoMs) and inhibited the polarization of MoMs into M2 macrophages, especially M2a and M2c. Furthermore, MSCs downregulated the excessive accumulation of Ly6c- monocytes in the lungs. While an intravenous adoptive transfer of Ly6c- monocytes promoted the infiltration of MoM and Th2 inflammation, that of MSC-exposed Ly6c- monocytes did not. Ex vivo Ly6c- MoMs upregulated M2-related genes, which were reduced by MSC treatment. Molecules secreted by Ly6c- MoMs from IL-13 Tg mice lungs upregulated the expression of fibrosis-related genes in fibroblasts, which were also suppressed by MSC treatment. In conclusion, intravenously administered MSCs attenuate asthma phenotypes of chronic asthma by modulating macrophages. Identifying M2 macrophage subtypes revealed that exposure to MSCs transforms the phenotype and function of macrophages. We suggest that Ly6c- monocytes could be a therapeutic target for asthma management.
Keywords: Asthma; Interleukin-13; Macrophages; Mesenchymal stem cells; Monocytes.
Copyright © 2022. The Korean Association of Immunologists.
Conflict of interest statement
Conflict of Interest: The authors declare no potential conflicts of interest.
Figures

Similar articles
-
Classical monocyte-derived macrophages as therapeutic targets of umbilical cord mesenchymal stem cells: comparison of intratracheal and intravenous administration in a mouse model of pulmonary fibrosis.Respir Res. 2023 Mar 5;24(1):68. doi: 10.1186/s12931-023-02357-x. Respir Res. 2023. PMID: 36870972 Free PMC article.
-
Intravenous Mesenchymal Stem Cell Administration Modulates Monocytes/Macrophages and Ameliorates Asthmatic Airway Inflammation in a Murine Asthma Model.Mol Cells. 2022 Nov 30;45(11):833-845. doi: 10.14348/molcells.2022.0038. Epub 2022 Nov 11. Mol Cells. 2022. PMID: 36380733 Free PMC article.
-
Mesenchymal stem cells exert their anti-asthmatic effects through macrophage modulation in a murine chronic asthma model.Sci Rep. 2022 Jun 13;12(1):9811. doi: 10.1038/s41598-022-14027-x. Sci Rep. 2022. PMID: 35697721 Free PMC article.
-
Mesenchymal stem cells influence monocyte/macrophage phenotype: Regulatory mode and potential clinical applications.Biomed Pharmacother. 2023 Sep;165:115042. doi: 10.1016/j.biopha.2023.115042. Epub 2023 Jun 26. Biomed Pharmacother. 2023. PMID: 37379639 Review.
-
Macrophages at the nexus of mesenchymal stromal cell potency: The emerging role of chemokine cooperativity.Stem Cells. 2021 Sep;39(9):1145-1154. doi: 10.1002/stem.3380. Epub 2021 Apr 6. Stem Cells. 2021. PMID: 33786935 Free PMC article. Review.
Cited by
-
Involvement of Transforming Growth Factor-β-Associated Kinase 1 in Fixed Airway Obstruction in Asthmatic Patients with Longer Disease Duration Independent on Airway Eosinophilia.J Asthma Allergy. 2023 Apr 4;16:343-354. doi: 10.2147/JAA.S403645. eCollection 2023. J Asthma Allergy. 2023. PMID: 37038432 Free PMC article.
-
Mesenchymal Stem/Stromal Cells in Asthma Therapy: Mechanisms and Strategies for Enhancement.Cell Transplant. 2023 Jan-Dec;32:9636897231180128. doi: 10.1177/09636897231180128. Cell Transplant. 2023. PMID: 37318186 Free PMC article. Review.
-
Mesenchymal Stem Cell-Derived Exosomes Attenuate Murine Cytomegalovirus-Infected Pneumonia via NF-κB/NLRP3 Signaling Pathway.Viruses. 2024 Apr 16;16(4):619. doi: 10.3390/v16040619. Viruses. 2024. PMID: 38675960 Free PMC article.
-
Immune Cell-Mediated Autoimmune Responses in Severe Asthma.Yonsei Med J. 2024 Apr;65(4):194-201. doi: 10.3349/ymj.2023.0432. Yonsei Med J. 2024. PMID: 38515356 Free PMC article. Review.
-
Inhibition of NADPH oxidase 2 enhances resistance to viral neuroinflammation by facilitating M1-polarization of macrophages at the extraneural tissues.J Neuroinflammation. 2024 May 2;21(1):115. doi: 10.1186/s12974-024-03078-8. J Neuroinflammation. 2024. PMID: 38698374 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

