IL-18 deficiency ameliorates the progression from AKI to CKD
- PMID: 36379914
- PMCID: PMC9666542
- DOI: 10.1038/s41419-022-05394-4
IL-18 deficiency ameliorates the progression from AKI to CKD
Abstract
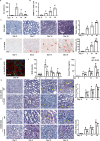
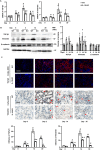
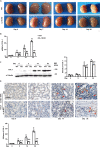
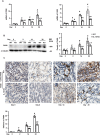
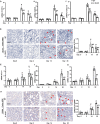
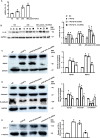
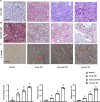
Inflammation is an important factor in the progression from acute kidney injury (AKI) to chronic kidney disease (CKD). The role of interleukin (IL)-18 in this progression has not been examined. We aimed to clarify whether and how IL-18 limits this progression. In a folic acid induced renal injury mouse model, we studied the time course of kidney injury and renal IL-18 expression. In wild-type mice following injection, renal IL-18 expression increased. In parallel, we characterized other processes, including at day 2, renal tubular necroptosis assessed by receptor-interacting serine/threonine-protein kinase1 (RIPK1) and RIPK3; at day 14, transdifferentiation (assessed by transforming growth factor β1, vimentin and E-cadherin); and at day 30, fibrosis (assessed by collagen 1). In IL-18 knockout mice given folate, compared to wild-type mice, tubular damage and necroptosis, transdifferentiation, and renal fibrosis were attenuated. Importantly, IL-18 deletion decreased numbers of renal M1 macrophages and M1 macrophage cytokine levels at day 14, and reduced M2 macrophages numbers and macrophage cytokine expression at day 30. In HK-2 cells, IL-18 knockdown attenuated necroptosis, transdifferentiating and fibrosis.In patients with tubulointerstitial nephritis, IL-18 protein expression was increased on renal biopsies using immunohistochemistry. We conclude that genetic IL-18 deficiency ameliorates renal tubular damage, necroptosis, cell transdifferentiation, and fibrosis. The renoprotective role of IL-18 deletion in the progression from AKI to fibrosis may be mediated by reducing a switch in predominance from M1 to profibrotic M2 macrophages during the process of kidney repair.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
IRF-4 deficiency reduces inflammation and kidney fibrosis after folic acid-induced acute kidney injury.Int Immunopharmacol. 2021 Nov;100:108142. doi: 10.1016/j.intimp.2021.108142. Epub 2021 Sep 20. Int Immunopharmacol. 2021. PMID: 34555644
-
RIPK3-MLKL-mediated necroinflammation contributes to AKI progression to CKD.Cell Death Dis. 2018 Aug 29;9(9):878. doi: 10.1038/s41419-018-0936-8. Cell Death Dis. 2018. PMID: 30158627 Free PMC article.
-
NFAT inhibitor 11R-VIVIT ameliorates mouse renal fibrosis after ischemia-reperfusion-induced acute kidney injury.Acta Pharmacol Sin. 2022 Aug;43(8):2081-2093. doi: 10.1038/s41401-021-00833-y. Epub 2021 Dec 22. Acta Pharmacol Sin. 2022. PMID: 34937917 Free PMC article.
-
Mechanisms and Models of Kidney Tubular Necrosis and Nephron Loss.J Am Soc Nephrol. 2022 Mar;33(3):472-486. doi: 10.1681/ASN.2021101293. Epub 2022 Jan 12. J Am Soc Nephrol. 2022. PMID: 35022311 Free PMC article. Review.
-
Macrophages polarization in renal inflammation and fibrosis animal models (Review).Mol Med Rep. 2024 Feb;29(2):29. doi: 10.3892/mmr.2023.13152. Epub 2023 Dec 22. Mol Med Rep. 2024. PMID: 38131228 Free PMC article. Review.
Cited by
-
Adenine-induced animal model of chronic kidney disease: current applications and future perspectives.Ren Fail. 2024 Dec;46(1):2336128. doi: 10.1080/0886022X.2024.2336128. Epub 2024 Apr 4. Ren Fail. 2024. PMID: 38575340 Free PMC article. Review.
-
Regulated necrosis role in inflammation and repair in acute kidney injury.Front Immunol. 2023 Nov 24;14:1324996. doi: 10.3389/fimmu.2023.1324996. eCollection 2023. Front Immunol. 2023. PMID: 38077379 Free PMC article. Review.
-
Biomarkers of chronic kidney disease in older individuals: navigating complexity in diagnosis.Front Med (Lausanne). 2024 Jul 11;11:1397160. doi: 10.3389/fmed.2024.1397160. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39055699 Free PMC article. Review.
-
Associations of systemic inflammatory regulators with CKD and kidney function: evidence from the bidirectional mendelian randomization study.BMC Nephrol. 2024 May 10;25(1):161. doi: 10.1186/s12882-024-03590-2. BMC Nephrol. 2024. PMID: 38730296 Free PMC article.
-
Gut microbiome and metabolome to discover pathogenic bacteria and probiotics in ankylosing spondylitis.Front Immunol. 2024 Apr 22;15:1369116. doi: 10.3389/fimmu.2024.1369116. eCollection 2024. Front Immunol. 2024. PMID: 38711505 Free PMC article.
References
-
- Ronco C, Bellomo R, Kellum J. Acute kidney injury. Lancet. 2019;394:1949–4. - PubMed
-
- Hoste E, Bagshaw S, Bellomo R, Cely C, Colman R, Cruz D, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–23. - PubMed
-
- Sato Y, Yanagita M. Immune cells and inflammation in AKI to CKD progression. Am J Physiol Ren Physiol. 2018;315:F1501–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

