Exercise training attenuates angiotensin II-induced cardiac fibrosis by reducing POU2F1 expression
- PMID: 36374849
- PMCID: PMC10362488
- DOI: 10.1016/j.jshs.2022.10.004
Exercise training attenuates angiotensin II-induced cardiac fibrosis by reducing POU2F1 expression
Abstract
Background: Exercise training protects against heart failure. However, the mechanism underlying the protective effect of exercise training on angiotensin II (Ang II)-induced cardiac fibrosis remains unclear.
Methods: An exercise model involving C57BL/6N mice and 6 weeks of treadmill training was used. Ang II (1.44 mg/kg/day) was administered to induce cardiac fibrosis. RNA sequencing and bioinformatic analysis were used to identify the key factors mediating the effects of exercise training on cardiac fibrosis. Primary adult mouse cardiac fibroblasts (CFs) were used in vitro. Adeno-associated virus serotype 9 was used to overexpress POU domain, class 2, transcription factor 1 (POU2F1) in vivo.
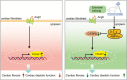
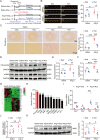
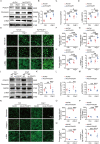
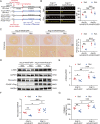
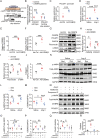
Results: Exercise training attenuated Ang II-induced cardiac fibrosis and reversed 39 gene expression changes. The transcription factor regulating the largest number of these genes was POU2F1. Compared to controls, POU2F1 was shown to be significantly upregulated by Ang II, which is itself reduced by exercise training. In vivo, POU2F1 overexpression nullified the benefits of exercise training on cardiac fibrosis. In CFs, POU2F1 promoted cardiac fibrosis. CCAAT enhancer-binding protein β (C/EBPβ) was predicted to be the transcription factor of POU2F1 and verified using a dual-luciferase reporter assay. In vivo, exercise training activated AMP-activated protein kinase (AMPK) and alleviated the increase in C/EBPβ induced by Ang II. In CFs, AMPK agonist inhibited the increase in C/EBPβ and POU2F1 induced by Ang II, whereas AMPK inhibitor reversed this effect.
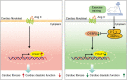
Conclusion: Exercise training attenuates Ang II-induced cardiac fibrosis by reducing POU2F1. Exercise training inhibits POU2F1 by activating AMPK, which is followed by the downregulation of C/EBPβ, the transcription factor of POU2F1.
Keywords: AMPK; C/EBPβ; Cardiac fibrosis; Exercise; POU2F1.
Copyright © 2022. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
[AMP-activated kinase activation inhibits transforming growth factor-β1 production in cardiac fibroblasts via targeting C/EBPβ].Sheng Li Xue Bao. 2017 Apr 25;69(2):123-128. Sheng Li Xue Bao. 2017. PMID: 28435970 Chinese.
-
Rhein attenuates angiotensin II-induced cardiac remodeling by modulating AMPK-FGF23 signaling.J Transl Med. 2022 Jul 6;20(1):305. doi: 10.1186/s12967-022-03482-9. J Transl Med. 2022. PMID: 35794561 Free PMC article.
-
Metformin attenuates angiotensin II-induced TGFβ1 expression by targeting hepatocyte nuclear factor-4-α.Br J Pharmacol. 2018 Apr;175(8):1217-1229. doi: 10.1111/bph.13753. Epub 2017 Mar 24. Br J Pharmacol. 2018. PMID: 28230250 Free PMC article.
-
SIRT2 Acts as a Cardioprotective Deacetylase in Pathological Cardiac Hypertrophy.Circulation. 2017 Nov 21;136(21):2051-2067. doi: 10.1161/CIRCULATIONAHA.117.028728. Epub 2017 Sep 25. Circulation. 2017. PMID: 28947430 Free PMC article.
-
Pathological matrix stiffness promotes cardiac fibroblast differentiation through the POU2F1 signaling pathway.Sci China Life Sci. 2021 Feb;64(2):242-254. doi: 10.1007/s11427-019-1747-y. Epub 2020 Jun 29. Sci China Life Sci. 2021. PMID: 32617828
Cited by
-
Exercise improves cardiac fibrosis by stimulating the release of endothelial progenitor cell-derived exosomes and upregulating miR-126 expression.Front Cardiovasc Med. 2024 May 9;11:1323329. doi: 10.3389/fcvm.2024.1323329. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38798919 Free PMC article. Review.
-
The Role and Underlying Mechanisms of Exercise in Heart Failure.Rev Cardiovasc Med. 2024 Aug 12;25(8):285. doi: 10.31083/j.rcm2508285. eCollection 2024 Aug. Rev Cardiovasc Med. 2024. PMID: 39228484 Free PMC article. Review.
References
-
- Milutinović K, Stojiljković S, Ćuk J, et al. Athlete's heart. Fizicka kultura. 2018;72:139–147.
-
- Villella M, Villella A. Exercise and cardiovascular diseases. Kidney Blood Press Res. 2014;39:147–153. - PubMed
-
- Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. - PubMed
-
- Orso F, Fabbri G, Maggioni AP. Epidemiology of heart failure. Handb Exp Pharmacol. 2017;243:15–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

