Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: Implications for neurodegenerative disease treatment
- PMID: 36372278
- PMCID: PMC9712272
- DOI: 10.1016/j.phrs.2022.106550
Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: Implications for neurodegenerative disease treatment
Abstract
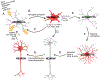
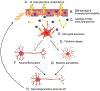
Chronic, excessive neuroinflammation is a key feature of neurodegenerative diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD). However, neuroinflammatory pathways have yet to be effectively targeted in clinical treatments for such diseases. Interestingly, increased inflammation and neurodegenerative disease risk have been associated with type 2 diabetes mellitus (T2DM) and insulin resistance (IR), suggesting that treatments that mitigate T2DM pathology may be successful in treating neuroinflammatory and neurodegenerative pathology as well. Glucagon-like peptide-1 (GLP-1) is an incretin hormone that promotes healthy insulin signaling, regulates blood sugar levels, and suppresses appetite. Consequently, numerous GLP-1 receptor (GLP-1R) stimulating drugs have been developed and approved by the US Food and Drug Administration (FDA) and related global regulatory authorities for the treatment of T2DM. Furthermore, GLP-1R stimulating drugs have been associated with anti-inflammatory, neurotrophic, and neuroprotective properties in neurodegenerative disorder preclinical models, and hence hold promise for repurposing as a treatment for neurodegenerative diseases. In this review, we discuss incretin signaling, neuroinflammatory pathways, and the intersections between neuroinflammation, brain IR, and neurodegenerative diseases, with a focus on AD and PD. We additionally overview current FDA-approved incretin receptor stimulating drugs and agents in development, including unimolecular single, dual, and triple receptor agonists, and highlight those in clinical trials for neurodegenerative disease treatment. We propose that repurposing already-approved GLP-1R agonists for the treatment of neurodegenerative diseases may be a safe, efficacious, and cost-effective strategy for ameliorating AD and PD pathology by quelling neuroinflammation.
Keywords: Alzheimer’s disease; Glucagon-like peptide-1 (GLP-1); Glucose-dependent insulinotropic peptide (GIP); Incretin mimetic; Insulin resistance; Neurodegeneration; Neuroinflammation; Parkinson’s disease.
Published by Elsevier Ltd.
Conflict of interest statement
Conflicts of interest NHG is an inventor on patents related to incretin mimetics and has assigned them in entirety to the National Institute on Aging, NIH, US Government, and hence has no personal rights to these patents. The National Institute on Aging, NIH, has a Cooperative Research and Development Agreement with Peptron Inc. (S. Korea) to support the evaluation of GLP-1R agonists in neurodegenerative disorders. All other authors declare no conflict of interest.
Figures
Similar articles
-
Incretin-Based Multi-Agonist Peptides Are Neuroprotective and Anti-Inflammatory in Cellular Models of Neurodegeneration.Biomolecules. 2024 Jul 19;14(7):872. doi: 10.3390/biom14070872. Biomolecules. 2024. PMID: 39062586 Free PMC article.
-
Incretin-based drugs as potential therapy for neurodegenerative diseases: current status and perspectives.Pharmacol Ther. 2022 Nov;239:108277. doi: 10.1016/j.pharmthera.2022.108277. Epub 2022 Sep 3. Pharmacol Ther. 2022. PMID: 36064147 Review.
-
Antidiabetic agents as a novel treatment for Alzheimer's and Parkinson's disease.Ageing Res Rev. 2023 Aug;89:101979. doi: 10.1016/j.arr.2023.101979. Epub 2023 Jun 14. Ageing Res Rev. 2023. PMID: 37328112 Review.
-
Glucagon-like peptide-1 (GLP-1)-based receptor agonists as a treatment for Parkinson's disease.Expert Opin Investig Drugs. 2020 Jun;29(6):595-602. doi: 10.1080/13543784.2020.1764534. Epub 2020 May 15. Expert Opin Investig Drugs. 2020. PMID: 32412796 Free PMC article. Review.
-
A New Treatment Strategy for Parkinson's Disease through the Gut-Brain Axis: The Glucagon-Like Peptide-1 Receptor Pathway.Cell Transplant. 2017 Sep;26(9):1560-1571. doi: 10.1177/0963689717721234. Cell Transplant. 2017. PMID: 29113464 Free PMC article. Review.
Cited by
-
Attenuating mitochondrial dysfunction and morphological disruption with PT320 delays dopamine degeneration in MitoPark mice.J Biomed Sci. 2024 Apr 17;31(1):38. doi: 10.1186/s12929-024-01025-6. J Biomed Sci. 2024. PMID: 38627765 Free PMC article.
-
Incretins and cardiovascular disease: to the heart of type 2 diabetes?Diabetologia. 2023 Oct;66(10):1820-1831. doi: 10.1007/s00125-023-05973-w. Epub 2023 Aug 5. Diabetologia. 2023. PMID: 37542009 Free PMC article. Review.
-
Type 2 diabetes mellitus/obesity drugs: A neurodegenerative disorders savior or a bridge too far?Ageing Res Rev. 2024 Jul;98:102343. doi: 10.1016/j.arr.2024.102343. Epub 2024 May 16. Ageing Res Rev. 2024. PMID: 38762101 Review.
-
Sitagliptin elevates plasma and CSF incretin levels following oral administration to nonhuman primates: relevance for neurodegenerative disorders.Geroscience. 2024 Oct;46(5):4397-4414. doi: 10.1007/s11357-024-01120-4. Epub 2024 Mar 27. Geroscience. 2024. PMID: 38532069 Free PMC article.
-
Noteworthy perspectives on microglia in neuropsychiatric disorders.J Neuroinflammation. 2023 Oct 4;20(1):223. doi: 10.1186/s12974-023-02901-y. J Neuroinflammation. 2023. PMID: 37794488 Free PMC article. Review.
References
-
- Bellenguez C, Kucukali F, Jansen IE, Kleineidam L, Moreno-Grau S, Amin N, Naj AC, Campos-Martin R, Grenier-Boley B, Andrade V, Holmans PA, Boland A, Damotte V, van der Lee SJ, Costa MR, Kuulasmaa T, Yang Q, De Rojas I, Bis JC, Yaqub A, Prokic I, Chapuis J, Ahmad S, Giedraitis V, Aarsland D, Garcia-Gonzalez P, Abdelnour C, Alarcon-Martin E, Alcolea D, Alegret M, Alvarez I, Alvarez V, Armstrong NJ, Tsolaki A, Antunez C, Appollonio I, Arcaro M, Archetti S, Pastor AA, Arosio B, Athanasiu L, Bailly H, Banaj N, Baquero M, Barral S, Beiser A, Pastor AB, Below JE, Benchek P, Benussi L, Berr C, Besse C, Bessi V, Binetti G, Bizarro A, Blesa R, Boada M, Boerwinkle E, Borroni B, Boschi S, Bossu P, Brathen G, Bressler J, Bresner C, Brodaty H, Brookes KJ, Brusco LI, Buiza-Rueda D, Burger K, Burholt V, Bush WS, Calero M, Cantwell LB, Chene G, Chung J, Cuccaro ML, Cecchetti R, Cervera-Carles L, Charbonnier C, Chen HH, Chillotti C, Ciccone S, Claassen JAHR, Clark C, Conti E, Corma-Gomez A, Costantini E, Custodero C, Daian D, Dalmasso MC, Daniele A, Dardiotis E, Dartigues JF, de Deyn PP, Lopes KD, De Witte LD, Debette S, Deckert J, del Ser T, Denning N, Destefano A, Dichgans M, Diehl-Schmid J, Diez-Fairen M, Rossi PD, Djurovic S, Duron E, Duzel E, Dufouil C, Eiriksdottir G, Engelborghs S, Escott-Price V, Espinosa A, Ewers M, Faber KM, Fabrizio T, Nielsen SF, Fardo DW, Farotti L, Fenoglio C, Fernandez-Fuertes M, Ferrari R, Ferreira CB, Ferri E, Fin B, Fischer P, Fladby T, Fliessbach K, Fongang B, Fornage M, Fortea J, Foroud TM, Fostinelli S, Fox NC, Franco-Macias E, Bullido MJ, Frank-Garcia A, Froelich L, Fulton-Howard B, Galimberti D, Garcia-Alberca JM, Garcia-Madrona S, Garcia-Ribas G, Ghidoni R, Giegling I, Giorgio G, Goate AM, Goldhardt O, Gomez-Fonseca D, Gonzalez-Perez A, Graff C, Grande G, Green E, Grimmer T, Grunblatt E, Grunin M, Gudnason V, Guetta-Baranes T, Haapasalo A, Hadjigeorgiou G, Haines JL, Hamilton-Nelson KL, Hampel H, Hanon O, Hardy J, Hartmann AM, Hausner L, Harwood J, Heilmann-Heimbach S, Helisalmi S, Heneka MT, Hernandez I, Herrmann MJ, Hoffmann P, Holmes C, Holstege H, Vilas RH, Hulsman M, Humphrey J, Biessels GJ, Jian XQ, Johansson C, Jun GR, Kastumata Y, Kauwe J, Kehoe PG, Kilander L, Stahlbom AK, Kivipelto M, Koivisto A, Kornhuber J, Kosmidis MH, Kukull WA, Kuksa PP, Kunkle BW, Kuzma AB, Lage C, Laukka EJ, Launer L, Lauria A, Lee CY, Lehtisalo J, Lerch O, Lleo A, Longstreth W, Lopez O, de Munain AL, Love S, Lowemark M, Luckcuck L, Lunetta KL, Ma YY, Macias J, Macleod CA, Maier W, Mangialasche F, Spallazzi M, Marquie M, Marshall R, Martin ER, Montes AM, Rodriguez CM, Masullo C, Mayeux R, Mead S, Mecocci P, Medina M, Meggy A, Mehrabian S, Mendoza S, Menendez-Gonzalez M, Mir P, Moebus S, Mol M, Molina-Porcel L, Montrreal L, Morelli L, Moreno F, Morgan K, Mosley T, Nothen MM, Muchnik C, Mukherjee S, Nacmias B, Ngandu T, Nicolas G, Nordestgaard BG, Olaso R, Orellana A, Orsini M, Ortega G, Padovani A, Paolo C, Papenberg G, Parnetti L, Pasquier F, Pastor P, Peloso G, Perez-Cordon A, Perez-Tur J, Pericard P, Peters O, Pijnenburg YAL, Pineda JA, Pinol-Ripoll G, Pisanu L, Polak T, Popp J, Posthuma D, Priller J, Puerta R, Quenez O, Quintela I, Thomassen JQ, Rabano A, Rainero I, Rajabli F, Ramakers I, Real LM, Reinders MJT, Reitz C, Reyes-Dumeyer D, Ridge P, Riedel-Heller S, Riederer P, Roberto N, Rodriguez-Rodriguez E, Rongve A, Allende IR, Rosende-Roca M, Royo JL, Rubino E, Rujescu D, Saez ME, Sakka P, Saltvedt I, Sanchez-Arjona MB, Sanchez-Garcia F, Juan PS, Sanchez-Valle R, Sando SB, Sarnowski C, Satizabal CL, Scamosci M, Scarmeas N, Scarpini E, Scheltens P, Scherbaum N, Scherer M, Schmid M, Schneider A, Schott JM, Selbaek G, Seripa D, Serrano M, Sha J, Shadrin AA, Skrobot O, Slifer S, Snijders GJL, Soininen H, Solfrizzi V, Solomon A, Song Y, Sorbi S, Sotolongo-Grau O, Spalletta G, Spottke A, Squassina A, Stordal E, Tartan JP, Tarraga L, Tesi N, Thalamuthu A, Thomas T, Tosto G, Traykov L, Tremolizzo L, Tybjaeg-Hansen A, Uitterlinden A, Ullgren A, Ulstein I, Valero S, Valladares O, Van Broeckhoven C, Vance J, Vardarajan BN, van der Lugt A, Van Dongen J, van Rooij J, van Swieten J, Vandenberghe R, Verhey F, Vidal JS, Vogelgsang J, Vyhnalek M, Wagner M, Wallon D, Wang LS, Wang RQ, Weinhold L, Wiltfang J, Windle G, Woods B, Yannakoulia M, Zare H, Zhao Y, Zhang XL, Zhu CC, Zulaica M, Farrer LA, Psaty BM, Ghanbari M, Raj T, Sachdev P, Mather K, Jessen F, Ikram MA, de Mendonca A, Hort J, Tsolaki M, Pericak-Vance MA, Amouyel P, Williams J, Frikke-Schmidt R, Clarimon J, Deleuze JF, Rossi G, Seshadri S, Andreassen OA, Ingelsson M, Hiltunen M, Sleegers K, Schellenberg GD, van Duijn CM, Sims R, van der Flier WM, Ruiz A, Ramirez A, Lambert JC, EADB, ACE G, DEGESCO, EADI, GERAD, Demgene, FinnGen, ADGC, CHARGE. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat Genet. 2022;54(4):412–+. - PMC - PubMed
-
- Marlet IR, Olmestig JNE, Vilsboll T, Rungby J, Kruuse C. Neuroprotective Mechanisms of Glucagon-like Peptide-1-based Therapies in Ischaemic Stroke: A Systematic Review based on Pre-Clinical Studies. Basic Clin Pharmacol Toxicol. 2018;122(6):559–569. - PubMed
-
- Malhotra K, Katsanos AH, Lambadiari V, Goyal N, Palaiodimou L, Kosmidou M, Krogias C, Alexandrov AV, Tsivgoulis G. GLP-1 receptor agonists in diabetes for stroke prevention: a systematic review and meta-analysis. J Neurol. 2020;267(7):2117–2122. - PubMed
-
- Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106(4):1285–1290. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

