Effect of deuteration on the single dose pharmacokinetic properties and postoperative analgesic activity of methadone
- PMID: 36368298
- PMCID: PMC9886271
- DOI: 10.1016/j.dmpk.2022.100477
Effect of deuteration on the single dose pharmacokinetic properties and postoperative analgesic activity of methadone
Abstract
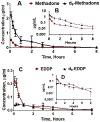
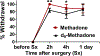
Although methadone is effective in the management of acute pain, the complexity of its absorption-distribution-metabolism-excretion profile limits its use as an opioid of choice for perioperative analgesia. Because deuteration is known to improve the pharmacokinetic, pharmacodynamic and toxicological properties of some drugs, here we characterized the single dose pharmacokinetic properties and post-operative analgesic efficacy of d9-methadone. The pharmacokinetic profiles of d9-methadone and methadone administered intravenously to CD-1 male mice revealed that deuteration leads to a 5.7- and 4.4-fold increase in the area under the time-concentration curve and maximum concentration in plasma, respectively, as well as reduction in clearance (0.9 ± 0.3 L/h/kg vs 4.7 ± 0.8 L/h/kg). The lower brain-to-plasma ratio of d9-methadone compared to that of methadone (0.35 ± 0.12 vs 2.05 ± 0.62) suggested that deuteration decreases the transfer of the drug across the blood-brain barrier. The estimated LD50 value for a single intravenous dose of d9-methadone was 2.1-fold higher than that for methadone. Moreover, d9-methadone outperformed methadone in the efficacy against postoperative pain by primarily activating peripheral opioid receptors. Collectively, these data suggest that the replacement of three hydrogen atoms in three methyl groups of methadone altered its pharmacokinetic properties, improved safety, and enhanced its analgesic efficacy.
Keywords: Deuteration; Metabolism; Pharmacokinetic; Postoperative pain; d(9)-methadone.
Copyright © 2022 The Japanese Society for the Study of Xenobiotics. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
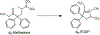
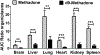

Similar articles
-
Can Saliva and Plasma Methadone Concentrations Be Used for Enantioselective Pharmacokinetic and Pharmacodynamic Studies in Patients With Advanced Cancer?Clin Ther. 2017 Sep;39(9):1840-1848. doi: 10.1016/j.clinthera.2017.07.044. Epub 2017 Aug 18. Clin Ther. 2017. PMID: 28827023 Clinical Trial.
-
[Methadone--pharmacokinetics and pharmacodynamics of an opiate].Anaesthesist. 1989 Apr;38(4):159-66. Anaesthesist. 1989. PMID: 2567128 Review. German.
-
A Novel Perioperative Multidose Methadone-Based Multimodal Analgesic Strategy in Children Achieved Safe and Low Analgesic Blood Methadone Levels Enabling Opioid-Sparing Sustained Analgesia With Minimal Adverse Effects.Anesth Analg. 2021 Aug 1;133(2):327-337. doi: 10.1213/ANE.0000000000005366. Anesth Analg. 2021. PMID: 33481403 Free PMC article. Clinical Trial.
-
The role of methadone in cancer pain treatment--a review.Int J Clin Pract. 2009 Jul;63(7):1095-109. doi: 10.1111/j.1742-1241.2008.01990.x. Int J Clin Pract. 2009. PMID: 19570126 Review.
-
Sex specificity in methadone analgesia in the rat: a population pharmacokinetic and pharmacodynamic approach.Pharm Res. 2002 Jun;19(6):858-67. doi: 10.1023/a:1016117218760. Pharm Res. 2002. PMID: 12134958
Cited by
-
Deuterium in drug discovery: progress, opportunities and challenges.Nat Rev Drug Discov. 2023 Jul;22(7):562-584. doi: 10.1038/s41573-023-00703-8. Epub 2023 Jun 5. Nat Rev Drug Discov. 2023. PMID: 37277503 Free PMC article. Review.
References
-
- Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Shear TD, Deshur MA, et al. Clinical Effectiveness and Safety of Intraoperative Methadone in Patients Undergoing Posterior Spinal Fusion Surgery: A Randomized, Double-blinded, Controlled Trial. Anesthesiology 2017;126(5):822–833. doi: 10.1097/ALN.0000000000001609 - DOI - PubMed
-
- Murphy GS, Szokol JW, Avram MJ, Greenberg SB, Marymont JH, Shear T, et al. Intraoperative Methadone for the Prevention of Postoperative Pain: A Randomized, Double-blinded Clinical Trial in Cardiac Surgical Patients. Anesthesiology 2015;122(5):1112–22. doi: 10.1097/ALN.0000000000000633 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

