How EBV Infects: The Tropism and Underlying Molecular Mechanism for Viral Infection
- PMID: 36366470
- PMCID: PMC9696472
- DOI: 10.3390/v14112372
How EBV Infects: The Tropism and Underlying Molecular Mechanism for Viral Infection
Abstract
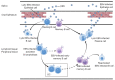
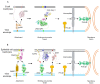
The Epstein-Barr virus (EBV) is associated with a variety of human malignancies, including Burkitt's lymphoma, Hodgkin's disease, nasopharyngeal carcinoma and gastric cancers. EBV infection is crucial for the oncogenesis of its host cells. The prerequisite for the establishment of infection is the virus entry. Interactions of viral membrane glycoproteins and host membrane receptors play important roles in the process of virus entry into host cells. Current studies have shown that the main tropism for EBV are B cells and epithelial cells and that EBV is also found in the tumor cells derived from NK/T cells and leiomyosarcoma. However, the process of EBV infecting B cells and epithelial cells significantly differs, relying on heterogenous glycoprotein-receptor interactions. This review focuses on the tropism and molecular mechanism of EBV infection. We systematically summarize the key molecular events that mediate EBV cell tropism and its entry into target cells and provide a comprehensive overview.
Keywords: Epstein–Barr virus (EBV); disease; entry; infection; tropism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Epstein-Barr virus-associated lymphoproliferative disorders.Postepy Hig Med Dosw (Online). 2013 May 24;67:481-90. doi: 10.5604/17322693.1050999. Postepy Hig Med Dosw (Online). 2013. PMID: 23752600 Review.
-
Membrane anchoring of Epstein-Barr virus gp42 inhibits fusion with B cells even with increased flexibility allowed by engineered spacers.mBio. 2015 Jan 6;6(1):e02285-14. doi: 10.1128/mBio.02285-14. mBio. 2015. PMID: 25564465 Free PMC article.
-
Epithelial cell infection by Epstein-Barr virus.FEMS Microbiol Rev. 2019 Nov 1;43(6):674-683. doi: 10.1093/femsre/fuz023. FEMS Microbiol Rev. 2019. PMID: 31584659 Free PMC article.
-
Epstein-Barr virus latency: LMP2, a regulator or means for Epstein-Barr virus persistence?Adv Cancer Res. 2000;79:175-200. doi: 10.1016/s0065-230x(00)79006-3. Adv Cancer Res. 2000. PMID: 10818681 Review.
-
Association between Antibody Responses to Epstein-Barr Virus Glycoproteins, Neutralization of Infectivity, and the Risk of Nasopharyngeal Carcinoma.mSphere. 2020 Dec 2;5(6):e00901-20. doi: 10.1128/mSphere.00901-20. mSphere. 2020. PMID: 33268566 Free PMC article.
Cited by
-
Natural killer cells and their exosomes in viral infections and related therapeutic approaches: where are we?Cell Commun Signal. 2023 Sep 25;21(1):261. doi: 10.1186/s12964-023-01266-2. Cell Commun Signal. 2023. PMID: 37749597 Free PMC article. Review.
-
Deciphering the Role of Epstein-Barr Virus Latent Membrane Protein 1 in Immune Modulation: A Multifaced Signalling Perspective.Viruses. 2024 Apr 4;16(4):564. doi: 10.3390/v16040564. Viruses. 2024. PMID: 38675906 Free PMC article. Review.
-
Research landmarks on the 60th anniversary of Epstein-Barr virus.Sci China Life Sci. 2025 Feb;68(2):354-380. doi: 10.1007/s11427-024-2766-0. Epub 2024 Nov 4. Sci China Life Sci. 2025. PMID: 39505801 Review.
-
EBV and Lymphomagenesis.Cancers (Basel). 2023 Apr 4;15(7):2133. doi: 10.3390/cancers15072133. Cancers (Basel). 2023. PMID: 37046794 Free PMC article. Review.
-
The role of EBV-encoded miRNA in EBV-associated gastric cancer.Front Oncol. 2023 Jun 14;13:1204030. doi: 10.3389/fonc.2023.1204030. eCollection 2023. Front Oncol. 2023. PMID: 37388232 Free PMC article. Review.
References
-
- WHO. International Agency for Research on Cancer . Epstein-Barr Virus and Kaposi’s Sarcoma Herpesvirus/Human Herpesvirus 8: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 70. IARC; Lyon, France: 1997. 492p - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

