Controlled Release of Bone Morphogenetic Protein-2 Augments the Coupling of Angiogenesis and Osteogenesis for Accelerating Mandibular Defect Repair
- PMID: 36365215
- PMCID: PMC9699026
- DOI: 10.3390/pharmaceutics14112397
Controlled Release of Bone Morphogenetic Protein-2 Augments the Coupling of Angiogenesis and Osteogenesis for Accelerating Mandibular Defect Repair
Abstract
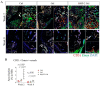
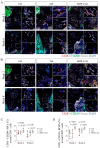
Reconstruction of a mandibular defect is challenging, with high expectations for both functional and esthetic results. Bone morphogenetic protein-2 (BMP-2) is an essential growth factor in osteogenesis, but the efficacy of the BMP-2-based strategy on the bone regeneration of mandibular defects has not been well-investigated. In addition, the underlying mechanisms of BMP-2 that drives the bone formation in mandibular defects remain to be clarified. Here, we utilized BMP-2-loaded hydrogel to augment bone formation in a critical-size mandibular defect model in rats. We found that implantation of BMP-2-loaded hydrogel significantly promoted intramembranous ossification within the defect. The region with new bone triggered by BMP-2 harbored abundant CD31+ endomucin+ type H vessels and associated osterix (Osx)+ osteoprogenitor cells. Intriguingly, the new bone comprised large numbers of skeletal stem cells (SSCs) (CD51+ CD200+) and their multi-potent descendants (CD51+ CD105+), which were mainly distributed adjacent to the invaded blood vessels, after implantation of the BMP-2-loaded hydrogel. Meanwhile, BMP-2 further elevated the fraction of CD51+ CD105+ SSC descendants. Overall, the evidence indicates that BMP-2 may recapitulate a close interaction between functional vessels and SSCs. We conclude that BMP-2 augmented coupling of angiogenesis and osteogenesis in a novel and indispensable way to improve bone regeneration in mandibular defects, and warrants clinical investigation and application.
Keywords: angiogenesis; bone morphogenetic protein-2; hydrogel; mandibular defect; osteogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Injectable temperature-sensitive hydrogel system incorporating deferoxamine-loaded microspheres promotes H-type blood vessel-related bone repair of a critical size femoral defect.Acta Biomater. 2022 Nov;153:108-123. doi: 10.1016/j.actbio.2022.09.018. Epub 2022 Sep 14. Acta Biomater. 2022. PMID: 36115651
-
Dual delivery of BMP-2 and bFGF from a new nano-composite scaffold, loaded with vascular stents for large-size mandibular defect regeneration.Int J Mol Sci. 2013 Jun 18;14(6):12714-28. doi: 10.3390/ijms140612714. Int J Mol Sci. 2013. PMID: 23778088 Free PMC article.
-
Effects of Bone Morphogenetic Protein-2 on Neovascularization During Large Bone Defect Regeneration.Tissue Eng Part A. 2019 Dec;25(23-24):1623-1634. doi: 10.1089/ten.TEA.2018.0326. Epub 2019 Jun 14. Tissue Eng Part A. 2019. PMID: 30973074 Free PMC article.
-
Delivery of the improved BMP-2-Advanced plasmid DNA within a gene-activated scaffold accelerates mesenchymal stem cell osteogenesis and critical size defect repair.J Control Release. 2018 Aug 10;283:20-31. doi: 10.1016/j.jconrel.2018.05.022. Epub 2018 May 19. J Control Release. 2018. PMID: 29782946
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
Cited by
-
Recent advancement in vascularized tissue-engineered bone based on materials design and modification.Mater Today Bio. 2023 Nov 11;23:100858. doi: 10.1016/j.mtbio.2023.100858. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 38024843 Free PMC article. Review.
-
Assessment of rhBMP-2-loaded bovine hydroxyapatite granules in the guided bone regeneration of critical bone defect in rat mandible bone.J Dent Sci. 2024 Jan;19(1):276-284. doi: 10.1016/j.jds.2023.04.016. Epub 2023 Apr 28. J Dent Sci. 2024. PMID: 38303875 Free PMC article. No abstract available.
-
SIRT1 activation promotes bone repair by enhancing the coupling of type H vessel formation and osteogenesis.Cell Prolif. 2024 Jun;57(6):e13596. doi: 10.1111/cpr.13596. Epub 2024 Jan 11. Cell Prolif. 2024. PMID: 38211965 Free PMC article.
-
Concentration-Dependent Efficacy of Recombinant Human Bone Morphogenetic Protein-2 Using a HA/β-TCP Hydrogel Carrier in a Mini-Pig Vertebral Oblique Lateral Interbody Fusion Model.Int J Mol Sci. 2023 Jan 3;24(1):892. doi: 10.3390/ijms24010892. Int J Mol Sci. 2023. PMID: 36614335 Free PMC article.
-
Use of Plant Extracts in Polymeric Scaffolds in the Regeneration of Mandibular Injuries.Pharmaceutics. 2024 Apr 2;16(4):491. doi: 10.3390/pharmaceutics16040491. Pharmaceutics. 2024. PMID: 38675152 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

