Efficient Synthesis of Fluorescent Coumarins and Phosphorous-Containing Coumarin-Type Heterocycles via Palladium Catalyzed Cross-Coupling Reactions
- PMID: 36364471
- PMCID: PMC9654183
- DOI: 10.3390/molecules27217649
Efficient Synthesis of Fluorescent Coumarins and Phosphorous-Containing Coumarin-Type Heterocycles via Palladium Catalyzed Cross-Coupling Reactions
Abstract
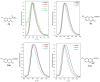
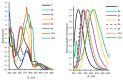
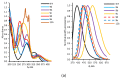
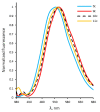
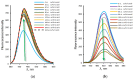
Quantum-chemical calculations on the spectral properties of some aryl substituted 3-phosphonocoumarins were performed, and the effect of the substituents in the aryl moiety was evaluated. The structures possessing promising fluorescent properties were successfully synthesized via Suzuki and Sonogashira cross-coupling. The synthetic protocol was also applied for the phosphorous chemoisomer of 3-phosphonocoumarin, 1,2-benzoxaphosphorin, and their carboxylate analogues. The optical properties of the arylated and alkynylated products were experimentally determined. The obtained quantum-chemical and experimental results give the possibility for a fine tuning of the optical properties of phosphorous-containing coumarin systems by altering the substituent at its C-6 position.
Keywords: 1,2-benzoxaphosphorin; 3-phosphonocoumarin; DFT calculations; Pd-catalyzed reactions; Sonogashira reaction; Suzuki reaction; coumarin-3-carboxylates; cross-coupling; fluorescence; photophysical properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
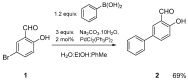
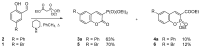
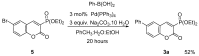
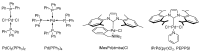
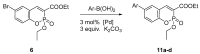
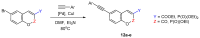


Similar articles
-
Sequential Pd(II)-Pd(0) catalysis for the rapid synthesis of coumarins.J Org Chem. 2005 Aug 5;70(16):6515-8. doi: 10.1021/jo050671l. J Org Chem. 2005. PMID: 16050721
-
Transition metal-catalyzed synthesis of new 3-substituted coumarin derivatives as antibacterial and cytostatic agents.Future Med Chem. 2021 Nov;13(21):1865-1884. doi: 10.4155/fmc-2021-0161. Epub 2021 Sep 17. Future Med Chem. 2021. PMID: 34533068
-
Palladium-catalyzed decarboxylative cross-coupling reactions: a route for regioselective functionalization of coumarins.J Org Chem. 2013 Apr 5;78(7):2957-64. doi: 10.1021/jo302778d. Epub 2013 Mar 11. J Org Chem. 2013. PMID: 23445254
-
Pd-catalyzed steroid reactions.Steroids. 2015 May;97:13-44. doi: 10.1016/j.steroids.2014.07.018. Epub 2014 Aug 27. Steroids. 2015. PMID: 25173819 Review.
-
Unique biomedical application of fluorescence derivatization based on palladium-catalyzed coupling reactions for HPLC analysis of pharmaceuticals and biomolecules.Biomed Chromatogr. 2024 Jul;38(7):e5857. doi: 10.1002/bmc.5857. Epub 2024 Mar 20. Biomed Chromatogr. 2024. PMID: 38509750 Review.
References
-
- Bhatia R., Pathania S., Singh V., Rawalet R.K. Metal-catalyzed synthetic strategies toward coumarin derivatives. Chem. Heterocycl. Comp. 2018;54:280–291. doi: 10.1007/s10593-018-2262-6. - DOI
-
- Szwaczko K. Coumarins Synthesis and Transformation via C–H Bond Activation—A Review. Inorganics. 2022;10:23. doi: 10.3390/inorganics10020023. - DOI
-
- Cheke R.S., Patel H.M., Patil V.M., Ansari I.A., Ambhore J.P., Shinde S.D., Kadri A., Snoussi M., Adnan M., Kharkar P.S., et al. Molecular Insights into Coumarin Analogues as Antimicrobial Agents: Recent Developments in Drug Discovery. Antibiotics. 2022;11:566. doi: 10.3390/antibiotics11050566. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

