Crosstalk between Blood Vessels and Glia during the Central Nervous System Development
- PMID: 36362915
- PMCID: PMC9699316
- DOI: 10.3390/life12111761
Crosstalk between Blood Vessels and Glia during the Central Nervous System Development
Abstract
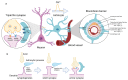
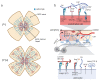
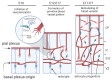
The formation of proper blood vessel patterns in the central nervous system (CNS) is crucial to deliver oxygen and nutrient to neurons efficiently. At the same time, neurons must be isolated from the outer blood circulation by a specialized structure, the blood-brain barrier (BBB), to maintain the microenvironment of brain parenchyma for the survival of neurons and proper synaptic transmission. To develop this highly organized structure, glial cells, a major component of the brain, have been reported to play essential roles. In this review, the crosstalk between the macroglia, including astrocytes and oligodendrocytes, and endothelial cells during the development of CNS will be discussed. First, the known roles of astrocytes in neuro-vascular unit and its development, and then, the requirements of astrocytes for BBB development and maintenance are shown. Then, various genetic and cellular studies revealing the roles of astrocytes in the growth of blood vessels by providing a scaffold, including laminins and fibronectin, as well as by secreting trophic factors, including vascular endothelial growth factor (VEGF) and transforming growth factor-β (TGF-β) are introduced. Finally, the interactions between oligodendrocyte progenitors and blood vessels are overviewed. Although these studies revealed the necessity for proper communication between glia and endothelial cells for CNS development, our knowledge about the detailed cellular and molecular mechanisms for them is still limited. The questions to be clarified in the future are also discussed.
Keywords: astrocyte; blood–brain barrier; oligodendrocyte.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Glial cells: an important switch for the vascular function of the central nervous system.Front Cell Neurosci. 2023 May 3;17:1166770. doi: 10.3389/fncel.2023.1166770. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37206667 Free PMC article. Review.
-
Radial Glia-endothelial Cells' Bidirectional Interactions Control Vascular Maturation and Astrocyte Differentiation: Impact for Blood-brain Barrier Formation.Curr Neurovasc Res. 2019;16(4):291-300. doi: 10.2174/1567202616666191014120156. Curr Neurovasc Res. 2019. PMID: 31633476
-
Neuro-glia interaction effects on GFAP gene: a novel role for transforming growth factor-beta1.Eur J Neurosci. 2002 Dec;16(11):2059-69. doi: 10.1046/j.1460-9568.2002.02283.x. Eur J Neurosci. 2002. PMID: 12473073
-
Glial-endothelial crosstalk regulates blood-brain barrier function.Curr Opin Pharmacol. 2016 Feb;26:39-46. doi: 10.1016/j.coph.2015.09.010. Epub 2015 Oct 19. Curr Opin Pharmacol. 2016. PMID: 26480201 Review.
-
Structure and Function of the Blood-Brain Barrier (BBB).Handb Exp Pharmacol. 2022;273:3-31. doi: 10.1007/164_2020_404. Handb Exp Pharmacol. 2022. PMID: 33249527
Cited by
-
Neuronal glycolysis: focus on developmental morphogenesis and localized subcellular functions.Commun Integr Biol. 2024 Apr 21;17(1):2343532. doi: 10.1080/19420889.2024.2343532. eCollection 2024. Commun Integr Biol. 2024. PMID: 38655369 Free PMC article. Review.
-
Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood-Brain Barrier.Cells. 2024 Jan 12;13(2):150. doi: 10.3390/cells13020150. Cells. 2024. PMID: 38247841 Free PMC article. Review.
-
Astrocytic Neuroimmunological Roles Interacting with Microglial Cells in Neurodegenerative Diseases.Int J Mol Sci. 2023 Jan 13;24(2):1599. doi: 10.3390/ijms24021599. Int J Mol Sci. 2023. PMID: 36675113 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

