Natriuretic Peptides-New Targets for Neurocontrol of Blood Pressure via Baroreflex Afferent Pathway
- PMID: 36362405
- PMCID: PMC9657840
- DOI: 10.3390/ijms232113619
Natriuretic Peptides-New Targets for Neurocontrol of Blood Pressure via Baroreflex Afferent Pathway
Abstract
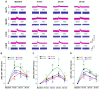
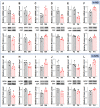
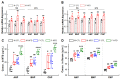
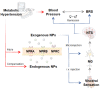
Natriuretic peptides (NPs) induce vasodilation, natriuresis, and diuresis, counteract the renin-angiotensin-aldosterone system and autonomic nervous system, and are key regulators of cardiovascular volume and pressure homeostasis. Baroreflex afferent pathway is an important reflex loop in the neuroregulation of blood pressure (BP), including nodose ganglion (NG) and nucleus tractus solitarius (NTS). Dysfunction of baroreflex would lead to various hypertensions. Here, we carried out functional experiments to explore the effects of NPs on baroreflex afferent function. Under physiological and hypertensive condition (high-fructose drinking-induced hypertension, HFD), BP was reduced by NPs through NG microinjection and baroreflex sensitivity (BRS) was enhanced via acute intravenous NPs injection. These anti-hypertensive effects were more obvious in female rats with the higher expression of NPs and its receptor A/B (NPRA/NPRB) and lower expression of its receptor C (NPRC). However, these effects were not as obvious as those in HFD rats compared with the same gender control group, which is likely to be explained by the abnormal expression of NPs and NPRs in the hypertensive condition. Our data provide additional evidence showing that NPs play a crucial role in neurocontrol of BP regulation via baroreflex afferent function and may be potential targets for clinical management of metabolic-related hypertension.
Keywords: baroreflex afferent function; blood pressure regulation; gender difference; high-fructose induced hypertension; natriuretic peptide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
FGF-21 ameliorates essential hypertension of SHR via baroreflex afferent function.Brain Res Bull. 2020 Jan;154:9-20. doi: 10.1016/j.brainresbull.2019.10.003. Epub 2019 Oct 15. Brain Res Bull. 2020. PMID: 31626954
-
Baroreflex afferent function is a part of insights of Leptin-mediated blood pressure reduction and Leptin-resistance hypertension.Neuropeptides. 2024 Jun;105:102418. doi: 10.1016/j.npep.2024.102418. Epub 2024 Feb 29. Neuropeptides. 2024. PMID: 38442503
-
The baroreflex afferent pathway plays a critical role in H2S-mediated autonomic control of blood pressure regulation under physiological and hypertensive conditions.Acta Pharmacol Sin. 2021 Jun;42(6):898-908. doi: 10.1038/s41401-020-00549-5. Epub 2020 Nov 5. Acta Pharmacol Sin. 2021. PMID: 33154555 Free PMC article.
-
Serotonin2 receptors in the nucleus tractus solitarius: characterization and role in the baroreceptor reflex arc.Cell Mol Neurobiol. 2003 Oct;23(4-5):709-26. doi: 10.1023/a:1025096718559. Cell Mol Neurobiol. 2003. PMID: 14514026 Review.
-
Molecular and genetic aspects of guanylyl cyclase natriuretic peptide receptor-A in regulation of blood pressure and renal function.Physiol Genomics. 2018 Nov 1;50(11):913-928. doi: 10.1152/physiolgenomics.00083.2018. Epub 2018 Aug 31. Physiol Genomics. 2018. PMID: 30169131 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

