COVID-19 Molecular Pathophysiology: Acetylation of Repurposing Drugs
- PMID: 36362045
- PMCID: PMC9656873
- DOI: 10.3390/ijms232113260
COVID-19 Molecular Pathophysiology: Acetylation of Repurposing Drugs
Abstract
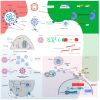
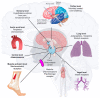
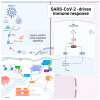
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) induces immune-mediated type 1 interferon (IFN-1) production, the pathophysiology of which involves sterile alpha motif and histidine-aspartate domain-containing protein 1 (SAMHD1) tetramerization and the cytosolic DNA sensor cyclic-GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling pathway. As a result, type I interferonopathies are exacerbated. Aspirin inhibits cGAS-mediated signaling through cGAS acetylation. Acetylation contributes to cGAS activity control and activates IFN-1 production and nuclear factor-κB (NF-κB) signaling via STING. Aspirin and dapsone inhibit the activation of both IFN-1 and NF-κB by targeting cGAS. We define these as anticatalytic mechanisms. It is necessary to alleviate the pathologic course and take the lag time of the odds of achieving viral clearance by day 7 to coordinate innate or adaptive immune cell reactions.
Keywords: ACE2 (angiotensin-converting enzyme 2); SAMHD1 (sterile alpha motif and histidine-aspartate domain-containing protein 1); SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2); TLR4 (Toll-like receptor 4); aspirin; cGAS–STING (cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) synthase (cGAS)–stimulator of interferon genes (STING)); dapsone; dexamethasone; immunologic engram; inflammasome; spike protein.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Treatment mechanism of immune triad from the repurposing drug against COVID-19.Transl Med Aging. 2023;7:33-45. doi: 10.1016/j.tma.2023.06.005. Epub 2023 Jun 24. Transl Med Aging. 2023. PMID: 37388715 Free PMC article. Review.
-
SARS-CoV-2, HIV, and HPV: Convergent evolution of selective regulation of cGAS-STING signaling.J Med Virol. 2023 Jan;95(1):e28220. doi: 10.1002/jmv.28220. Epub 2022 Oct 26. J Med Virol. 2023. PMID: 36229923 Free PMC article. Review.
-
Salmonella Induces the cGAS-STING-Dependent Type I Interferon Response in Murine Macrophages by Triggering mtDNA Release.mBio. 2022 Jun 28;13(3):e0363221. doi: 10.1128/mbio.03632-21. Epub 2022 May 23. mBio. 2022. PMID: 35604097 Free PMC article.
-
Aggravating mechanisms from COVID-19.Virol J. 2024 Sep 27;21(1):228. doi: 10.1186/s12985-024-02506-8. Virol J. 2024. PMID: 39334442 Free PMC article. Review.
-
Role of micronucleus-activated cGAS-STING signaling in antitumor immunity.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Jan 26;53(1):25-34. doi: 10.3724/zdxbyxb-2023-0485. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38273467 Free PMC article. Review. Chinese, English.
Cited by
-
Advanced Therapy Medicinal Products as Potential Therapeutic Strategy against COVID-19 and Immune-Related Disorders.Int J Mol Sci. 2024 Mar 6;25(5):3079. doi: 10.3390/ijms25053079. Int J Mol Sci. 2024. PMID: 38474324 Free PMC article.
-
At the Crossroads of the cGAS-cGAMP-STING Pathway and the DNA Damage Response: Implications for Cancer Progression and Treatment.Pharmaceuticals (Basel). 2023 Dec 1;16(12):1675. doi: 10.3390/ph16121675. Pharmaceuticals (Basel). 2023. PMID: 38139802 Free PMC article. Review.
-
Approaching Personalized Medicine: The Use of Machine Learning to Determine Predictors of Mortality in a Population with SARS-CoV-2 Infection.Biomedicines. 2024 Feb 9;12(2):409. doi: 10.3390/biomedicines12020409. Biomedicines. 2024. PMID: 38398012 Free PMC article.
-
Treatment mechanism of immune triad from the repurposing drug against COVID-19.Transl Med Aging. 2023;7:33-45. doi: 10.1016/j.tma.2023.06.005. Epub 2023 Jun 24. Transl Med Aging. 2023. PMID: 37388715 Free PMC article. Review.
-
COVID-19 and Diarylamidines: The Parasitic Connection.Int J Mol Sci. 2023 Apr 1;24(7):6583. doi: 10.3390/ijms24076583. Int J Mol Sci. 2023. PMID: 37047556 Free PMC article. Review.
References
-
- Rodrigues T.S., de Sá K.S.G., Ishimoto A.Y., Becerra A., Oliveira S., Almeida L., Gonçalves A.V., Perucello D.B., Andrade W.A., Castro R., et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2020;218:e20201707. doi: 10.1084/jem.20201707. - DOI - PMC - PubMed
-
- Saris A., Reijnders T.D.Y., Nossent E.J., Schuurman A.R., Verhoeff J., van Asten S., Bontkes H., Blok S., Duitman J., Bogaard H.-J., et al. Distinct cellular immune profiles in the airways and blood of critically ill patients with COVID-19. Thorax. 2021;76:1010–1019. doi: 10.1136/thoraxjnl-2020-216256. - DOI - PMC - PubMed
-
- Wauters E., Van Mol P., Garg A.D., Jansen S., Van Herck Y., Vanderbeke L., Bassez A., Boeckx B., Malengier-Devlies B., Timmerman A., et al. Discriminating Mild from Critical COVID-19 by Innate and Adaptive Immune Single-cell Profiling of Bronchoalveolar Lavages. BioRxiv. 2021;31:272–290. doi: 10.1038/s41422-020-00455-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

