Amyloid Beta in Aging and Alzheimer's Disease
- PMID: 36361714
- PMCID: PMC9655207
- DOI: 10.3390/ijms232112924
Amyloid Beta in Aging and Alzheimer's Disease
Abstract
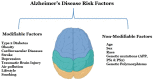
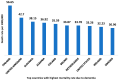
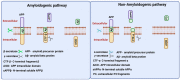
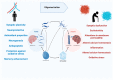
Alzheimer's disease (AD), is a progressive neurodegenerative disease that affects behavior, thinking, learning, and memory in elderly individuals. AD occurs in two forms, early onset familial and late-onset sporadic; genetic mutations in PS1, PS2, and APP genes cause early onset familial AD, and a combination of lifestyle, environment and genetic factors causes the late-onset sporadic form of the disease. However, accelerated disease progression is noticed in patients with familial AD. Disease-causing pathological changes are synaptic damage, and mitochondrial structural and functional changes, in addition to increased production and accumulation of phosphorylated tau (p-tau), and amyloid beta (Aβ) in the affected brain regions in AD patients. Aβ is a peptide derived from amyloid precursor protein (APP) by proteolytic cleavage of beta and gamma secretases. APP is a glycoprotein that plays a significant role in maintaining neuronal homeostasis like signaling, neuronal development, and intracellular transport. Aβ is reported to have both protective and toxic effects in neurons. The purpose of our article is to summarize recent developments of Aβ and its association with synapses, mitochondria, microglia, astrocytes, and its interaction with p-tau. Our article also covers the therapeutic strategies that reduce Aβ toxicities in disease progression and discusses the reasons for the failures of Aβ therapeutics.
Keywords: Alzheimer’s disease; amyloid beta; amyloid precursor protein; mitochondria; neurofibrillary tangle; therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Molecular Basis of Alzheimer's Disease: Focus on Mitochondria.J Alzheimers Dis. 2019;72(s1):S95-S116. doi: 10.3233/JAD-190048. J Alzheimers Dis. 2019. PMID: 30932888 Review.
-
APP/PS1 mice overexpressing SREBP-2 exhibit combined Aβ accumulation and tau pathology underlying Alzheimer's disease.Hum Mol Genet. 2013 Sep 1;22(17):3460-76. doi: 10.1093/hmg/ddt201. Epub 2013 May 6. Hum Mol Genet. 2013. PMID: 23648430 Free PMC article.
-
Modeling Alzheimer's disease in mouse without mutant protein overexpression: cooperative and independent effects of Aβ and tau.PLoS One. 2013 Nov 20;8(11):e80706. doi: 10.1371/journal.pone.0080706. eCollection 2013. PLoS One. 2013. PMID: 24278307 Free PMC article.
-
Regional distribution of synaptic markers and APP correlate with distinct clinicopathological features in sporadic and familial Alzheimer's disease.Brain. 2014 May;137(Pt 5):1533-49. doi: 10.1093/brain/awu046. Epub 2014 Mar 12. Brain. 2014. PMID: 24625695 Free PMC article.
Cited by
-
The past and present of therapeutic strategy for Alzheimer's diseases: potential for stem cell therapy.Exp Anim. 2023 Aug 7;72(3):285-293. doi: 10.1538/expanim.22-0164. Epub 2023 Mar 6. Exp Anim. 2023. PMID: 36878603 Free PMC article. Review.
-
The orchestrating role of deteriorating neurons and TREM-1 in crosstalk with SYK in Alzheimer's disease progression and neuroinflammation.Inflammopharmacology. 2023 Oct;31(5):2303-2310. doi: 10.1007/s10787-023-01270-5. Epub 2023 Jul 5. Inflammopharmacology. 2023. PMID: 37405587 Review.
-
A systematic review of salivary biomarkers in Parkinson's disease.Neural Regen Res. 2024 Dec 1;19(12):2613-2625. doi: 10.4103/NRR.NRR-D-23-01677. Epub 2024 Mar 1. Neural Regen Res. 2024. PMID: 38595280 Free PMC article.
-
Potential Application of MicroRNAs and Some Other Molecular Biomarkers in Alzheimer's Disease.Curr Issues Mol Biol. 2024 May 22;46(6):5066-5084. doi: 10.3390/cimb46060304. Curr Issues Mol Biol. 2024. PMID: 38920976 Free PMC article. Review.
-
From Plaques to Pathways in Alzheimer's Disease: The Mitochondrial-Neurovascular-Metabolic Hypothesis.Int J Mol Sci. 2024 Oct 31;25(21):11720. doi: 10.3390/ijms252111720. Int J Mol Sci. 2024. PMID: 39519272 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

