Review of the Existing Translational Pharmacokinetics Modeling Approaches Specific to Monoclonal Antibodies (mAbs) to Support the First-In-Human (FIH) Dose Selection
- PMID: 36361546
- PMCID: PMC9657028
- DOI: 10.3390/ijms232112754
Review of the Existing Translational Pharmacokinetics Modeling Approaches Specific to Monoclonal Antibodies (mAbs) to Support the First-In-Human (FIH) Dose Selection
Abstract
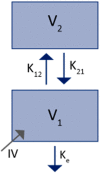
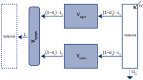
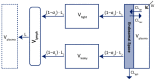
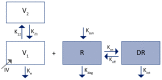
The interest in therapeutic monoclonal antibodies (mAbs) has continuously growing in several diseases. However, their pharmacokinetics (PK) is complex due to their target-mediated drug disposition (TMDD) profiles which can induce a non-linear PK. This point is particularly challenging during the pre-clinical and translational development of a new mAb. This article reviews and describes the existing PK modeling approaches used to translate the mAbs PK from animal to human for intravenous (IV) and subcutaneous (SC) administration routes. Several approaches are presented, from the most empirical models to full physiologically based pharmacokinetic (PBPK) models, with a focus on the population PK methods (compartmental and minimal PBPK models). They include the translational approaches for the linear part of the PK and the TMDD mechanism of mAbs. The objective of this article is to provide an up-to-date overview and future perspectives of the translational PK approaches for mAbs during a model-informed drug development (MIDD), since the field of PK modeling has gained recently significant interest for guiding mAbs drug development.
Keywords: ADA; PBPK; PopPK; first-in-human; mPBPK; modeling; monoclonal antibody; pharmacokinetics; translational.
Conflict of interest statement
Blaise Pasquiers & Mathieu Felices are employed by PhinC Development (CRO). Laurent Nguyen is employed by Sanofi.
Figures

Similar articles
-
Translation of Monoclonal Antibodies Pharmacokinetics from Animal to Human Using Physiologically Based Modeling in Open Systems Pharmacology (OSP) Suite: A Retrospective Analysis of Bevacizumab.Pharmaceutics. 2023 Aug 14;15(8):2129. doi: 10.3390/pharmaceutics15082129. Pharmaceutics. 2023. PMID: 37631343 Free PMC article.
-
Projecting human pharmacokinetics of monoclonal antibodies from nonclinical data: comparative evaluation of prediction approaches in early drug development.Biopharm Drug Dispos. 2016 Mar;37(2):51-65. doi: 10.1002/bdd.1952. Epub 2015 May 15. Biopharm Drug Dispos. 2016. PMID: 25869767 Review.
-
Understanding the Monoclonal Antibody Disposition after Subcutaneous Administration using a Minimal Physiologically based Pharmacokinetic Model.J Pharm Pharm Sci. 2018;21(1s):130s-148s. doi: 10.18433/jpps30028. J Pharm Pharm Sci. 2018. PMID: 30011390 Free PMC article.
-
Predicting monoclonal antibody pharmacokinetics following subcutaneous administration via whole-body physiologically-based modeling.J Pharmacokinet Pharmacodyn. 2020 Oct;47(5):385-409. doi: 10.1007/s10928-020-09691-3. Epub 2020 Jun 4. J Pharmacokinet Pharmacodyn. 2020. PMID: 32500362 Free PMC article.
-
Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies.Clin Pharmacokinet. 2013 Feb;52(2):83-124. doi: 10.1007/s40262-012-0027-4. Clin Pharmacokinet. 2013. PMID: 23299465 Review.
Cited by
-
Modeling the subcutaneous pharmacokinetics of antibodies co-administered with rHuPH20.Clin Transl Sci. 2024 Apr;17(4):e13788. doi: 10.1111/cts.13788. Clin Transl Sci. 2024. PMID: 38561908 Free PMC article.
-
Pharmacokinetic Models of Tafenoquine: Insights for Optimal Malaria Treatment Strategies.Pharmaceutics. 2024 Aug 26;16(9):1124. doi: 10.3390/pharmaceutics16091124. Pharmaceutics. 2024. PMID: 39339162 Free PMC article. Review.
-
Trial watch: chemotherapy-induced immunogenic cell death in oncology.Oncoimmunology. 2023 Jun 3;12(1):2219591. doi: 10.1080/2162402X.2023.2219591. eCollection 2023. Oncoimmunology. 2023. PMID: 37284695 Free PMC article. Review.
-
PD-L1-negative Non-small-cell Lung Cancer Treated with Nivolumab Plus Ipilimumab during Maintenance Hemodialysis Results in Rapid Initial Progression Followed by a Long-lasting Response.Intern Med. 2024 Apr 1;63(7):985-988. doi: 10.2169/internalmedicine.2270-23. Epub 2023 Aug 9. Intern Med. 2024. PMID: 37558475 Free PMC article.
-
Analysis of the pharmacokinetics and efficacy of RBD1016 - A GalNAc-siRNA targeting Hepatitis B Virus X gene using semi-mechanistic PK/PD model.Heliyon. 2024 May 24;10(11):e31924. doi: 10.1016/j.heliyon.2024.e31924. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38841435 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

