Brain Tumor Networks in Diffuse Glioma
- PMID: 36357661
- PMCID: PMC9723066
- DOI: 10.1007/s13311-022-01320-w
Brain Tumor Networks in Diffuse Glioma
Abstract
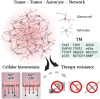
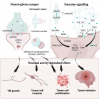
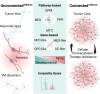
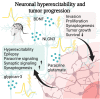
Diffuse gliomas are primary brain tumors associated with a poor prognosis. Cellular and molecular mechanisms driving the invasive growth patterns and therapeutic resistance are incompletely understood. The emerging field of cancer neuroscience offers a novel approach to study these brain tumors in the context of their intricate interactions with the nervous system employing and combining methodological toolsets from neuroscience and oncology. Increasing evidence has shown how neurodevelopmental and neuronal-like mechanisms are hijacked leading to the discovery of multicellular brain tumor networks. Here, we review how gap junction-coupled tumor-tumor-astrocyte networks, as well as synaptic and paracrine neuron-tumor networks drive glioma progression. Molecular mechanisms of these malignant, homo- and heterotypic networks, and their complex interplay are reviewed. Lastly, potential clinical-translational implications and resulting therapeutic strategies are discussed.
Keywords: Cancer neuroscience; Diffuse glioma; Glioblastoma; Neuron-glioma synapse; Neuron-tumor networks; Tumor-tumor networks.
© 2022. The Author(s).
Figures
Similar articles
-
Central nervous system regulation of diffuse glioma growth and invasion: from single unit physiology to circuit remodeling.J Neurooncol. 2024 Aug;169(1):1-10. doi: 10.1007/s11060-024-04719-x. Epub 2024 Jun 4. J Neurooncol. 2024. PMID: 38834748 Free PMC article. Review.
-
Neuron-Glial Interactions in Health and Brain Cancer.Adv Biol (Weinh). 2022 Sep;6(9):e2200122. doi: 10.1002/adbi.202200122. Epub 2022 Aug 11. Adv Biol (Weinh). 2022. PMID: 35957525 Free PMC article. Review.
-
Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment.J Natl Cancer Inst. 2007 Nov 7;99(21):1583-93. doi: 10.1093/jnci/djm187. Epub 2007 Oct 30. J Natl Cancer Inst. 2007. PMID: 17971532 Review.
-
Recapitulating in vivo-like plasticity of glioma cell invasion along blood vessels and in astrocyte-rich stroma.Histochem Cell Biol. 2017 Oct;148(4):395-406. doi: 10.1007/s00418-017-1604-2. Epub 2017 Aug 19. Histochem Cell Biol. 2017. PMID: 28825130 Free PMC article.
-
Synaptic input to brain tumors: clinical implications.Neuro Oncol. 2021 Jan 30;23(1):23-33. doi: 10.1093/neuonc/noaa158. Neuro Oncol. 2021. PMID: 32623467 Free PMC article.
Cited by
-
Tumor Microenvironment Modulation by Cancer-Derived Extracellular Vesicles.Cells. 2024 Apr 15;13(8):682. doi: 10.3390/cells13080682. Cells. 2024. PMID: 38667297 Free PMC article. Review.
-
The immune system and metabolic products in epilepsy and glioma-associated epilepsy: emerging therapeutic directions.JCI Insight. 2024 Jan 9;9(1):e174753. doi: 10.1172/jci.insight.174753. JCI Insight. 2024. PMID: 38193532 Free PMC article. Review.
-
Ion Channels and Ionotropic Receptors in Astrocytes: Physiological Functions and Alterations in Alzheimer's Disease and Glioblastoma.Life (Basel). 2023 Oct 11;13(10):2038. doi: 10.3390/life13102038. Life (Basel). 2023. PMID: 37895420 Free PMC article. Review.
-
Remarks on Selected Morphological Aspects of Cancer Neuroscience: A Microscopic Photo Review.Biomedicines. 2024 Oct 14;12(10):2335. doi: 10.3390/biomedicines12102335. Biomedicines. 2024. PMID: 39457647 Free PMC article. Review.
-
Therapeutic Advances in Neuro-Oncology.Neurotherapeutics. 2022 Oct;19(6):1689-1690. doi: 10.1007/s13311-022-01326-4. Neurotherapeutics. 2022. PMID: 36344725 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

