Andrographolide Promotes Uptake of Glucose and GLUT4 Transport through the PKC Pathway in L6 Cells
- PMID: 36355518
- PMCID: PMC9698946
- DOI: 10.3390/ph15111346
Andrographolide Promotes Uptake of Glucose and GLUT4 Transport through the PKC Pathway in L6 Cells
Abstract
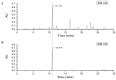
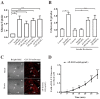
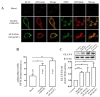
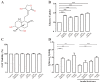
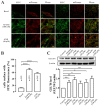
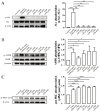
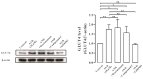
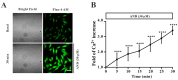
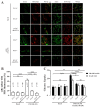
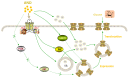
Glucose transporter 4 (GLUT4) is a membrane protein that regulates blood glucose balance and is closely related to type 2 diabetes. Andrographolide (AND) is a diterpene lactone extracted from herbal medicine Andrographis paniculata, which has a variety of biological activities. In this study, the antidiabetic effect of AND in L6 cells and its mechanism were investigated. The uptake of glucose of L6 cells was detected by a glucose assay kit. The expression of GLUT4 and phosphorylation of protein kinase B (PKB/Akt), AMP-dependent protein kinase (AMPK), and protein kinase C (PKC) were detected by Western blot. At the same time, the intracellular Ca2+ levels and GLUT4 translocation in myc-GLUT4-mOrange-L6 cells were detected by confocal laser scanning microscopy. The results showed that AND enhanced the uptake of glucose, GLUT4 expression and fusion with plasma membrane in L6 cells. Meanwhile, AND also significantly activated the phosphorylation of AMPK and PKC and increased the concentration of intracellular Ca2+. AND-induced GLUT4 expression was significantly inhibited by a PKC inhibitor (Gö6983). In addition, in the case of 0 mM extracellular Ca2+ and 0 mM extracellular Ca2+ + 10 μM BAPTA-AM (intracellular Ca2+ chelator), AND induced the translocation of GLUT4, and the uptake of glucose was significantly inhibited. Therefore, we concluded that AND promoted the expression of GLUT4 and its fusion with plasma membrane in L6 cells through PKC pathways in a Ca2+-dependent manner, thereby increasing the uptake of glucose.
Keywords: Ca2+; GLUT4; L6 cells; andrographolide; type 2 diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Ethanolic Extract of Folium Sennae Mediates the Glucose Uptake of L6 Cells by GLUT4 and Ca2.Molecules. 2018 Nov 9;23(11):2934. doi: 10.3390/molecules23112934. Molecules. 2018. PMID: 30424024 Free PMC article.
-
Dandelion Chloroform Extract Promotes Glucose Uptake via the AMPK/GLUT4 Pathway in L6 Cells.Evid Based Complement Alternat Med. 2018 Nov 7;2018:1709587. doi: 10.1155/2018/1709587. eCollection 2018. Evid Based Complement Alternat Med. 2018. PMID: 30524480 Free PMC article.
-
Glucose Uptake Is Increased by Estradiol Dipropionate in L6 Skeletal Muscle Cells.Pharmaceuticals (Basel). 2022 Dec 25;16(1):25. doi: 10.3390/ph16010025. Pharmaceuticals (Basel). 2022. PMID: 36678522 Free PMC article.
-
Neferine Promotes GLUT4 Expression and Fusion With the Plasma Membrane to Induce Glucose Uptake in L6 Cells.Front Pharmacol. 2019 Sep 4;10:999. doi: 10.3389/fphar.2019.00999. eCollection 2019. Front Pharmacol. 2019. PMID: 31551792 Free PMC article.
-
Chloroquine Increases Glucose Uptake via Enhancing GLUT4 Translocation and Fusion with the Plasma Membrane in L6 Cells.Cell Physiol Biochem. 2016;38(5):2030-40. doi: 10.1159/000445562. Epub 2016 May 9. Cell Physiol Biochem. 2016. PMID: 27160165
Cited by
-
Effect of Black Tea Polysaccharides on Alleviating Type 2 Diabetes Mellitus by Regulating PI3K/Akt/GLUT2 Pathway.Foods. 2024 Jun 17;13(12):1908. doi: 10.3390/foods13121908. Foods. 2024. PMID: 38928848 Free PMC article.
-
Terminalia bellirica and Andrographis paniculata dietary supplementation in mitigating heat stress-induced behavioral, metabolic and genetic alterations in broiler chickens.BMC Vet Res. 2024 Sep 3;20(1):388. doi: 10.1186/s12917-024-04233-2. BMC Vet Res. 2024. PMID: 39227945 Free PMC article.
References
-
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

