The identification of the key residues E829 and R845 involved in transient receptor potential melastatin 2 channel gating
- PMID: 36353687
- PMCID: PMC9637858
- DOI: 10.3389/fnagi.2022.1033434
The identification of the key residues E829 and R845 involved in transient receptor potential melastatin 2 channel gating
Abstract
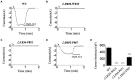
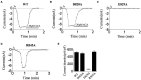
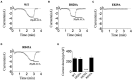
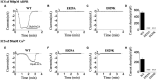
Transient receptor potential melastatin 2 (TRPM2), a non-selective cation channel, is involved in many physiological and pathological processes, including temperature sensing, synaptic plasticity regulation, and neurodegenerative diseases. However, the gating mechanism of TRPM2 channel is complex, which hinders its functional research. With the discovery of the Ca2+ binding site in the S2-S3 domain of TRPM2 channel, more and more attention has been drawn to the role of the transmembrane segments in channel gating. In this study, we focused on the D820-F867 segment around the S2 domain, and identified the key residues on it. Functional assays of the deletion mutants displayed that the deletions of D820-W835 and L836-P851 destroyed channel function totally, indicating the importance of these two segments. Sequence alignments on them found three polar and charged residues with high conservation (D820, E829, and R845). D820A, E829A, and R845A which removed the charge and the side chain of the residues were tested by 500 μM adenosine diphosphate-ribose (ADPR) or 50 mM Ca2+. E829A and R845A affected the characteristic of channel currents, while D820A behaved similarly to WT, indicating the participations of E829 and R845 in channel gating. The charge reversing mutants, E829K and R845D were then constructed and the electrophysiological tests showed that E829A and E829K made the channel lose function. Interestingly, R845A and R845D exhibited an inactivation process when using 500 μM ADPR, but activated normally by 50 mM Ca2+. Our data suggested that the negative charge at E829 took a vital part in channel activation, and R845 increased the stability of the Ca2+ combination in S2-S3 domain, thus guaranteeing the opening of TRPM2 channel. In summary, our identification of the key residues E829 and R845 in the transmembrane segments of TRPM2. By exploring the gating process of TRPM2 channel, our work helps us better understand the mechanism of TRPM2 as a potential biomarker in neurodegenerative diseases, and provides a new approach for the prediction, diagnosis, and prognosis of neurodegenerative diseases.
Keywords: TRPM2; activation; gating mechanism; inactivation; key residues; transmembrane segments.
Copyright © 2022 Luo, Chen, Wu, Jiang and Fang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Effects of calcium-binding sites in the S2-S3 loop on human and Nematostella vectensis TRPM2 channel gating processes.J Zhejiang Univ Sci B. 2019 Dec.;20(12):972-982. doi: 10.1631/jzus.B1900477. J Zhejiang Univ Sci B. 2019. PMID: 31749344 Free PMC article.
-
Sites of the NUDT9-H domain critical for ADP-ribose activation of the cation channel TRPM2.J Biol Chem. 2004 Nov 5;279(45):46431-7. doi: 10.1074/jbc.M407263200. Epub 2004 Sep 3. J Biol Chem. 2004. PMID: 15347676
-
Intracellular calcium activates TRPM2 and its alternative spliced isoforms.Proc Natl Acad Sci U S A. 2009 Apr 28;106(17):7239-44. doi: 10.1073/pnas.0811725106. Epub 2009 Apr 16. Proc Natl Acad Sci U S A. 2009. PMID: 19372375 Free PMC article.
-
Mechanism of TRPM2 channel gating revealed by cryo-EM.FEBS J. 2019 Sep;286(17):3333-3339. doi: 10.1111/febs.14939. Epub 2019 Jun 10. FEBS J. 2019. PMID: 31144442 Free PMC article. Review.
-
TRPM2: a multifunctional ion channel for calcium signalling.J Physiol. 2011 Apr 1;589(Pt 7):1515-25. doi: 10.1113/jphysiol.2010.201855. Epub 2010 Dec 6. J Physiol. 2011. PMID: 21135052 Free PMC article. Review.
References
-
- El Ghaleb Y., Schneeberger P. E., Fernandez-Quintero M. L., Geisler S. M., Pelizzari S., Polstra A. M., et al. . (2021). CACNA1I gain-of-function mutations differentially affect channel gating and cause neurodevelopmental disorders. Brain 144, 2092–2106. doi: 10.1093/brain/awab101, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

