m6A demethylase FTO renders radioresistance of nasopharyngeal carcinoma via promoting OTUB1-mediated anti-ferroptosis
- PMID: 36343416
- PMCID: PMC9646990
- DOI: 10.1016/j.tranon.2022.101576
m6A demethylase FTO renders radioresistance of nasopharyngeal carcinoma via promoting OTUB1-mediated anti-ferroptosis
Abstract
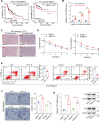
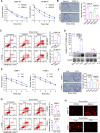
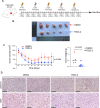
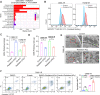
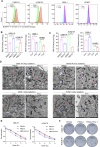
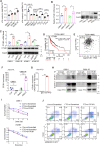
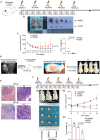
Radiotherapy is a valid treatment for nasopharyngeal carcinoma (NPC), and radioresistance is the main cause of local NPC treatment failure. However, the underlying mechanisms and valuable markers of radioresistance for NPC remain have not been established. In this study, we observed that the m6A mRNA demethylase fat mass and obesity-associated protein (FTO) was significantly upregulated in radioresistant NPC tissues and cells relative to parental radiosensitive NPC tissues and cells. FTO enhances radioresistance by repressing radiation-induced ferroptosis in NPC. Mechanistically, FTO acts as an m6A demethylase to erase the m6A modification of the OTUB1 transcript and promote the expression of OTUB1, thereby inhibiting the ferroptosis of cells induced by radiation and finally triggering the radiotherapy resistance of NPC. Furthermore, our in vivo experiment results showed that the FTO inhibitor, FB23-2, and the ferroptosis activator, erastin, altered tumor responsiveness to radiotherapy in NPC cell lines and patient-derived xenografts. Our findings reveal, for the first time, that FTO enhances NPC radiotherapy resistance by withstanding radiation-induced ferroptosis, suggesting that FTO may serve as a potential therapeutic target and valuable prognostic biomarker in patients with NPC.
Keywords: Nasopharyngeal carcinoma (NPC), FTO, Ferroptosis; OTUB1; m6A.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no competing interests.
Figures
Similar articles
-
LncRNA HOTAIRM1 promotes radioresistance in nasopharyngeal carcinoma by modulating FTO acetylation-dependent alternative splicing of CD44.Neoplasia. 2024 Oct;56:101034. doi: 10.1016/j.neo.2024.101034. Epub 2024 Aug 10. Neoplasia. 2024. PMID: 39128424 Free PMC article.
-
Itraconazole attenuates the stemness of nasopharyngeal carcinoma cells via triggering ferroptosis.Environ Toxicol. 2021 Feb;36(2):257-266. doi: 10.1002/tox.23031. Epub 2020 Sep 20. Environ Toxicol. 2021. PMID: 32951314
-
The m6A demethylases FTO and ALKBH5 aggravate the malignant progression of nasopharyngeal carcinoma by coregulating ARHGAP35.Cell Death Discov. 2024 Jan 23;10(1):43. doi: 10.1038/s41420-024-01810-0. Cell Death Discov. 2024. PMID: 38263362 Free PMC article.
-
FTO m6A Demethylase in Obesity and Cancer: Implications and Underlying Molecular Mechanisms.Int J Mol Sci. 2022 Mar 30;23(7):3800. doi: 10.3390/ijms23073800. Int J Mol Sci. 2022. PMID: 35409166 Free PMC article. Review.
-
Research progress on m6A demethylase FTO and its role in gynecological tumors.Front Oncol. 2024 Aug 8;14:1413505. doi: 10.3389/fonc.2024.1413505. eCollection 2024. Front Oncol. 2024. PMID: 39175477 Free PMC article. Review.
Cited by
-
Role of N6-methyladenosine methylation in head and neck cancer and its regulation of innate immune pathways.Front Immunol. 2024 Sep 30;15:1458884. doi: 10.3389/fimmu.2024.1458884. eCollection 2024. Front Immunol. 2024. PMID: 39403369 Free PMC article. Review.
-
Fat Mass- and Obesity-Associated Protein (FTO) Promotes the Proliferation of Goat Skeletal Muscle Satellite Cells by Stabilizing DAG1 mRNA in an IGF2BP1-Related m6A Manner.Int J Mol Sci. 2024 Sep 11;25(18):9804. doi: 10.3390/ijms25189804. Int J Mol Sci. 2024. PMID: 39337293 Free PMC article.
-
LncRNA HOTAIRM1 promotes radioresistance in nasopharyngeal carcinoma by modulating FTO acetylation-dependent alternative splicing of CD44.Neoplasia. 2024 Oct;56:101034. doi: 10.1016/j.neo.2024.101034. Epub 2024 Aug 10. Neoplasia. 2024. PMID: 39128424 Free PMC article.
-
OTUB1 regulates ferroptosis to inhibit myoblast differentiation into myotubes by deubiquitinating P62.Sci Rep. 2024 Jul 8;14(1):15696. doi: 10.1038/s41598-024-66868-3. Sci Rep. 2024. PMID: 38977909 Free PMC article.
-
FTO deficiency in older livers exacerbates ferroptosis during ischaemia/reperfusion injury by upregulating ACSL4 and TFRC.Nat Commun. 2024 Jun 4;15(1):4760. doi: 10.1038/s41467-024-49202-3. Nat Commun. 2024. PMID: 38834654 Free PMC article.
References
-
- Wong K., et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat. Rev. Clin. Oncol. 2021;18(11):679–695. - PubMed
-
- Ou D., et al. Induction chemotherapy with docetaxel, cisplatin and fluorouracil followed by concurrent chemoradiotherapy or chemoradiotherapy alone in locally advanced non-endemic nasopharyngeal carcinoma. Oral Oncol. 2016;62:114–121. - PubMed
-
- Bossi P., et al. Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021;32(4):452–465. official journal of the European Society for Medical Oncology. - PubMed
-
- Au K., et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: A report of 3328 patients (HKNPCSG 1301 study) Oral Oncol. 2018;77:16–21. - PubMed
-
- Moon S., et al. IMRT vs. 2D-radiotherapy or 3D-conformal radiotherapy of nasopharyngeal carcinoma: Survival outcome in a Korean multi-institutional retrospective study (KROG 11-06) Strahlenther. Onkol. 2016;192(6):377–385. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

