The role of sulfur compounds in chronic obstructive pulmonary disease
- PMID: 36339716
- PMCID: PMC9626809
- DOI: 10.3389/fmolb.2022.928287
The role of sulfur compounds in chronic obstructive pulmonary disease
Abstract
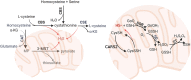
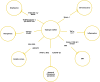
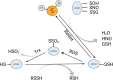
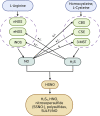
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease that brings about great social and economic burden, with oxidative stress and inflammation affecting the whole disease progress. Sulfur compounds such as hydrogen sulfide (H2S), thiols, and persulfides/polysulfides have intrinsic antioxidant and anti-inflammatory ability, which is engaged in the pathophysiological process of COPD. Hydrogen sulfide mainly exhibits its function by S-sulfidation of the cysteine residue of the targeted proteins. It also interacts with nitric oxide and acts as a potential biomarker for the COPD phenotype. Thiols' redox buffer such as the glutathione redox couple is a major non-enzymatic redox buffer reflecting the oxidative stress in the organism. The disturbance of redox buffers was often detected in patients with COPD, and redressing the balance could delay COPD exacerbation. Sulfane sulfur refers to a divalent sulfur atom bonded with another sulfur atom. Among them, persulfides and polysulfides have an evolutionarily conserved modification with antiaging effects. Sulfur compounds and their relative signaling pathways are also associated with the development of comorbidities in COPD. Synthetic compounds which can release H2S and persulfides in the organism have gradually been developed. Naturally extracted sulfur compounds with pharmacological effects also aroused great interest. This study discussed the biological functions and mechanisms of sulfur compounds in regulating COPD and its comorbidities.
Keywords: chronic obstructive pulmonary disease; hydrogen sulfide; sulfane sulfur; sulfur compound; thiols.
Copyright © 2022 Jiang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Fluorescent probes for hydrogen sulfide (H2S) and sulfane sulfur and their applications to biological studies.Nitric Oxide. 2015 Apr 30;46:72-9. doi: 10.1016/j.niox.2014.11.008. Epub 2014 Nov 13. Nitric Oxide. 2015. PMID: 25461270 Review.
-
Sulfane sulfur - new findings on an old topic.Acta Biochim Pol. 2019 Dec 28;66(4):533-544. doi: 10.18388/abp.2019_2909. Acta Biochim Pol. 2019. PMID: 31883321 Review.
-
Chemical tools for the study of hydrogen sulfide (H2S) and sulfane sulfur and their applications to biological studies.J Clin Biochem Nutr. 2016 Jan;58(1):7-15. doi: 10.3164/jcbn.15-91. Epub 2015 Dec 8. J Clin Biochem Nutr. 2016. PMID: 26798192 Free PMC article. Review.
-
The multifaceted roles of sulfane sulfur species in cancer-associated processes.Biochim Biophys Acta Bioenerg. 2021 Feb 1;1862(2):148338. doi: 10.1016/j.bbabio.2020.148338. Epub 2020 Nov 17. Biochim Biophys Acta Bioenerg. 2021. PMID: 33212042 Review.
-
Cysteine Metabolism and Oxidative Processes in the Rat Liver and Kidney after Acute and Repeated Cocaine Treatment.PLoS One. 2016 Jan 25;11(1):e0147238. doi: 10.1371/journal.pone.0147238. eCollection 2016. PLoS One. 2016. PMID: 26808533 Free PMC article.
Cited by
-
Effects of condensates from volcanic fumaroles and cigarette smoke extracts on airway epithelial cells.Hum Cell. 2023 Sep;36(5):1689-1702. doi: 10.1007/s13577-023-00927-1. Epub 2023 Jun 12. Hum Cell. 2023. PMID: 37308740 Free PMC article.
-
Hydrogen Sulfide: An Emerging Regulator of Oxidative Stress and Cellular Homeostasis-A Comprehensive One-Year Review.Antioxidants (Basel). 2023 Sep 7;12(9):1737. doi: 10.3390/antiox12091737. Antioxidants (Basel). 2023. PMID: 37760041 Free PMC article. Review.
-
Hydrogen Sulfide Improves Outcomes in a Murine Model of Necrotizing Enterocolitis via the Cys440 Residue on Endothelial Nitric Oxide Synthase.J Pediatr Surg. 2023 Dec;58(12):2391-2398. doi: 10.1016/j.jpedsurg.2023.08.006. Epub 2023 Aug 18. J Pediatr Surg. 2023. PMID: 37684170 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

