Histological regression of peritoneal metastases of recurrent tubo-ovarian cancer after systemic chemotherapy
- PMID: 36338656
- PMCID: PMC9632969
- DOI: 10.3389/fsurg.2022.936613
Histological regression of peritoneal metastases of recurrent tubo-ovarian cancer after systemic chemotherapy
Erratum in
-
Erratum: Histological regression of peritoneal metastases of recurrent tuboovarian cancer after systemic chemotherapy.Front Surg. 2023 Mar 9;10:1175506. doi: 10.3389/fsurg.2023.1175506. eCollection 2023. Front Surg. 2023. PMID: 36969755 Free PMC article.
Abstract
Introduction: Post-treatment histological regression of peritoneal metastases (PM) is a new and potentially important predictor of oncological outcomes. Histology of PM from adnexal origin is usually evaluated by the Chemotherapy Response Score (CRS). The aim of this preliminary study was to quantify the response of PM of recurrent tubo-ovarian cancer (TOVC) after systemic chemotherapy by using the recently validated Peritoneal Regression Grading System (PRGS) and compare it with CRS. Correlation with per operative evaluation through Peritoneal Cancer Index (PCI) was performed.
Material and methods: Retrospective cohort study of all consecutive patients with recurrent PM from TOVC undergoing surgery after prior systemic chemotherapy from January 2015 to March 2019. Biopsies were assessed with the four-scale PRGS.
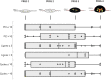
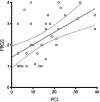
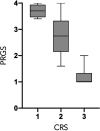
Results: Thirty-eight patients were included. Patients had a median of 2 (range 1-2) lines and 12 (range 3-18) cycles of prior systemic chemotherapy. Overall mean (SD) PRGS was 2.3 (±1.1). Of the patients, 26% (10) had complete response (PRGS 1), 40% (15) had major response (PRGS 2), 26% (10) minor response (PRGS 3), and 8% (3) had no response (PRGS 4). Mean PRGS was positively correlated with the Peritoneal Cancer Index (ρ = 0.5302, p = 0.0003) and inversely correlated with CRS (ρ = -0.8403, p < 0.0001). No correlation was highlighted between mean PRGS and overall survival (ρ = -0.0195, p = 0.9073).
Conclusion: CRS and mean PRGS correlated with each other. Histological response of PM after systemic chemotherapy was quantifiable and variable. The role of PRGS for the evaluation of treatment response and as potential surrogate marker for oncological outcomes is part of ongoing and planned research.
Keywords: PRGS; chemotherapy; gynecology; histology; ovary; peritoneal metastasis; surgery.
© 2022 Pache, Teixeira Farinha, Toussaint, Demartines, Hastir, Mathevet, Sempoux and Hübner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Histological regression of gastrointestinal peritoneal metastases after systemic chemotherapy.Pleura Peritoneum. 2021 Jul 15;6(3):113-119. doi: 10.1515/pp-2021-0118. eCollection 2021 Sep. Pleura Peritoneum. 2021. PMID: 34676284 Free PMC article.
-
Impact of preoperative chemotherapy on the histological response of patients with peritoneal metastases from colorectal cancer according to peritoneal regression grading score (PRGS) and TRG.Surg Oncol. 2020 Jun;33:158-163. doi: 10.1016/j.suronc.2020.02.014. Epub 2020 Feb 15. Surg Oncol. 2020. PMID: 32561082
-
Peritoneal regression grading score (PRGS) in peritoneal metastasis: how many biopsies should be examined?Pleura Peritoneum. 2022 Sep 26;7(4):179-185. doi: 10.1515/pp-2022-0118. eCollection 2022 Dec. Pleura Peritoneum. 2022. PMID: 36560968 Free PMC article.
-
Response Evaluation in Patients with Peritoneal Metastasis Treated with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC).J Clin Med. 2023 Feb 6;12(4):1289. doi: 10.3390/jcm12041289. J Clin Med. 2023. PMID: 36835824 Free PMC article. Review.
-
Severe peritoneal sclerosis after repeated pressurized intraperitoneal aerosol chemotherapy with oxaliplatin (PIPAC OX): report of two cases and literature survey.Clin Exp Metastasis. 2018 Mar;35(3):103-108. doi: 10.1007/s10585-018-9895-9. Epub 2018 Apr 28. Clin Exp Metastasis. 2018. PMID: 29705882 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

