The amplification of CNS damage in Alzheimer's disease due to SARS-CoV2 infection
- PMID: 36334414
- PMCID: PMC9616485
- DOI: 10.1016/j.anndiagpath.2022.152057
The amplification of CNS damage in Alzheimer's disease due to SARS-CoV2 infection
Abstract
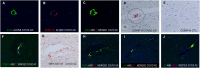
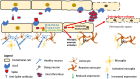
Pre-existing Alzheimer's disease is a risk factor for severe/fatal COVID-19 and infection by SARS-CoV2 virus has been associated with an increased incidence of un-masked Alzheimer's disease. The molecular basis whereby SARS-CoV2 may amplify Alzheimer's disease is not well understood. This study analyzed the molecular changes in autopsy brain tissues from people with pre-existing dementia who died of COVID-19 (n = 5) which was compared to equivalent tissues of people who died of COVID-19 with no history of dementia (n = 8), Alzheimer's disease pre-COVID-19 (n = 10) and aged matched controls (n = 10) in a blinded fashion. Immunohistochemistry analyses for hyperphosphorylated tau protein, α-synuclein, and β-amyloid-42 confirmed the diagnoses of Alzheimer's disease (n = 4), and Lewy body dementia (n = 1) in the COVID-19 group. The brain tissues from patients who died of COVID-19 with no history of dementia showed a diffuse microangiopathy marked by endocytosis of spike subunit S1 and S2 in primarily CD31+ endothelia with strong co-localization with ACE2, Caspase-3, IL6, TNFα, and Complement component 6 that was not associated with SARS-CoV2 RNA. Microglial activation marked by increased TMEM119 and MCP1 protein expression closely paralleled the endocytosed spike protein. The COVID-19 tissues from people with no pre-existing dementia showed, compared to controls, 5-10× fold increases in expression of neuronal NOS and NMDAR2 as well as a marked decrease in the expression of proteins whose loss is associated with worsening Alzheimer's disease: MFSD2a, SHIP1, BCL6, BCL10, and BACH1. In COVID-19 tissues from people with dementia the widespread spike-induced microencephalitis with the concomitant microglial activation co-existed in the same areas where neurons had hyperphosphorylated tau protein suggesting that the already dysfunctional neurons were additionally stressed by the SARS-CoV2 induced microangiopathy. ACE2+ human brain endothelial cells treated with high dose (but not vaccine equivalent low dose) spike S1 protein demonstrated each of the molecular changes noted in the in vivo COVID-19 and COVID-19/Alzheimer's disease brain tissues. It is concluded that fatal COVID-19 induces a diffuse microencephalitis and microglial activation in the brain due to endocytosis of circulating viral spike protein that amplifies pre-existing dementia in at least two ways: 1) modulates the expression of proteins that may worsen Alzheimer's disease and 2) stresses the already dysfunctional neurons by causing an acute proinflammatory/hypercoagulable/hypoxic microenvironment in areas with abundant hyperphosphorylated tau protein and/or βA-42.
Keywords: COVID-19; Encephalitis; Endothelialitis; Microvasculature; Spike Protein.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no conflicts of interest to disclose.
Figures




Similar articles
-
Endothelial cell damage is the central part of COVID-19 and a mouse model induced by injection of the S1 subunit of the spike protein.Ann Diagn Pathol. 2021 Apr;51:151682. doi: 10.1016/j.anndiagpath.2020.151682. Epub 2020 Dec 24. Ann Diagn Pathol. 2021. PMID: 33360731 Free PMC article.
-
The Effects of Aβ1-42 Binding to the SARS-CoV-2 Spike Protein S1 Subunit and Angiotensin-Converting Enzyme 2.Int J Mol Sci. 2021 Jul 30;22(15):8226. doi: 10.3390/ijms22158226. Int J Mol Sci. 2021. PMID: 34360989 Free PMC article.
-
Histologic, viral, and molecular correlates of heart disease in fatal COVID-19.Ann Diagn Pathol. 2022 Oct;60:151983. doi: 10.1016/j.anndiagpath.2022.151983. Epub 2022 May 29. Ann Diagn Pathol. 2022. PMID: 35660807 Free PMC article.
-
Exploring the Paradox of COVID-19 in Neurological Complications with Emphasis on Parkinson's and Alzheimer's Disease.Oxid Med Cell Longev. 2022 Aug 31;2022:3012778. doi: 10.1155/2022/3012778. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36092161 Free PMC article. Review.
-
The Future of the COVID-19 Pandemic: How Good (or Bad) Can the SARS-CoV2 Spike Protein Get?Cells. 2022 Mar 2;11(5):855. doi: 10.3390/cells11050855. Cells. 2022. PMID: 35269476 Free PMC article. Review.
Cited by
-
The association between rs6859 in NECTIN2 gene and Alzheimer's disease is partly mediated by pTau.medRxiv [Preprint]. 2024 Jun 22:2024.06.21.24309310. doi: 10.1101/2024.06.21.24309310. medRxiv. 2024. Update in: Front Aging Neurosci. 2024 Aug 06;16:1388363. doi: 10.3389/fnagi.2024.1388363. PMID: 38947013 Free PMC article. Updated. Preprint.
-
Refueling the post COVID-19 brain: potential role of ketogenic medium chain triglyceride supplementation: an hypothesis.Front Nutr. 2023 Jun 21;10:1126534. doi: 10.3389/fnut.2023.1126534. eCollection 2023. Front Nutr. 2023. PMID: 37415915 Free PMC article.
-
'Spikeopathy': COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA.Biomedicines. 2023 Aug 17;11(8):2287. doi: 10.3390/biomedicines11082287. Biomedicines. 2023. PMID: 37626783 Free PMC article. Review.
-
Impact of the SARS-CoV-2 Spike Protein on the Innate Immune System: A Review.Cureus. 2024 Mar 26;16(3):e57008. doi: 10.7759/cureus.57008. eCollection 2024 Mar. Cureus. 2024. PMID: 38549864 Free PMC article. Review.
-
The Neuropathological Impacts of COVID-19: Challenges and Alternative Treatment Options for Alzheimer's Like Brain Changes on Severely SARS-CoV-2 Infected Patients.Am J Alzheimers Dis Other Demen. 2023 Jan-Dec;38:15333175231214974. doi: 10.1177/15333175231214974. Am J Alzheimers Dis Other Demen. 2023. PMID: 37972355 Free PMC article. Review.
References
-
- Abbasi J. The COVID heart—one year after SARS-CoV-2 infection, patients have an Array of increased cardiovascular risks. JAMA. 2022;327:1113–1114. - PubMed
-
- AboTaleb H.A. Neurological complications in COVID-19 patients and its implications for associated mortality. Curr Neurovasc Res. 2020;17:522–530. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

