The multitaskers of the brain: Glial responses to viral infections and associated post-infectious neurologic sequelae
- PMID: 36334073
- PMCID: PMC9931640
- DOI: 10.1002/glia.24294
The multitaskers of the brain: Glial responses to viral infections and associated post-infectious neurologic sequelae
Abstract
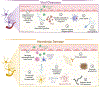
Many viral infections cause acute and chronic neurologic diseases which can lead to degeneration of cortical functions. While neurotropic viruses that gain access to the central nervous system (CNS) may induce brain injury directly via infection of neurons or their supporting cells, they also alter brain function via indirect neuroimmune mechanisms that may disrupt the blood-brain barrier (BBB), eliminate synapses, and generate neurotoxic astrocytes and microglia that prevent recovery of neuronal circuits. Non-neuroinvasive, neurovirulent viruses may also trigger aberrant responses in glial cells, including those that interfere with motor and sensory behaviors, encoding of memories and executive function. Increasing evidence from human and animal studies indicate that neuroprotective antiviral responses that amplify levels of innate immune molecules dysregulate normal neuroimmune processes, even in the absence of neuroinvasion, which may persist after virus is cleared. In this review, we discuss how select emerging and re-emerging RNA viruses induce neuroimmunologic responses that lead to dysfunction of higher order processes including visuospatial recognition, learning and memory, and motor control. Identifying therapeutic targets that return the neuroimmune system to homeostasis is critical for preventing virus-induced neurodegenerative disorders.
Keywords: astrocyte; host-pathogen interactions; microglia; neuroimmunology; neurovirology.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
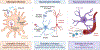
Similar articles
-
Viral Encephalitis and Neurologic Diseases: Focus on Astrocytes.Trends Mol Med. 2018 Nov;24(11):950-962. doi: 10.1016/j.molmed.2018.09.001. Epub 2018 Oct 9. Trends Mol Med. 2018. PMID: 30314877 Free PMC article. Review.
-
How viral infections cause neuronal dysfunction: a focus on the role of microglia and astrocytes.Biochem Soc Trans. 2023 Feb 27;51(1):259-274. doi: 10.1042/BST20220771. Biochem Soc Trans. 2023. PMID: 36606670 Review.
-
Immune response and blood-brain barrier dysfunction during viral neuroinvasion.Innate Immun. 2021 Feb;27(2):109-117. doi: 10.1177/1753425920954281. Epub 2020 Sep 9. Innate Immun. 2021. PMID: 32903111 Free PMC article. Review.
-
Neuroinflammation During RNA Viral Infections.Annu Rev Immunol. 2019 Apr 26;37:73-95. doi: 10.1146/annurev-immunol-042718-041417. Annu Rev Immunol. 2019. PMID: 31026414 Free PMC article. Review.
-
Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals.mBio. 2014 Aug 26;5(5):e01476-14. doi: 10.1128/mBio.01476-14. mBio. 2014. PMID: 25161189 Free PMC article.
Cited by
-
Fibromyalgia is associated with hypersensitivity but not with abnormal pain modulation: evidence from QST trials and spinal fMRI.Front Pain Res (Lausanne). 2023 Dec 5;4:1284103. doi: 10.3389/fpain.2023.1284103. eCollection 2023. Front Pain Res (Lausanne). 2023. PMID: 38116188 Free PMC article. Review.
-
Arbovirus infection increases the risk for the development of neurodegenerative disease pathology in the murine model.Brain Behav Immun Health. 2024 Apr 24;38:100780. doi: 10.1016/j.bbih.2024.100780. eCollection 2024 Jul. Brain Behav Immun Health. 2024. PMID: 38706571 Free PMC article.
-
DYT-TOR1A dystonia: an update on pathogenesis and treatment.Front Neurosci. 2023 Aug 10;17:1216929. doi: 10.3389/fnins.2023.1216929. eCollection 2023. Front Neurosci. 2023. PMID: 37638318 Free PMC article. Review.
-
Live-attenuated Japanese encephalitis virus inhibits glioblastoma growth and elicits potent antitumor immunity.Front Immunol. 2023 Apr 11;14:982180. doi: 10.3389/fimmu.2023.982180. eCollection 2023. Front Immunol. 2023. PMID: 37114043 Free PMC article.
References
-
- Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, … Steves CJ (2022). Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. The Lancet Infectious Diseases, 22(1), 43–55. doi: 10.1016/S1473-3099(21)00460-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

