Glial progenitor cells of the adult human white and grey matter are contextually distinct
- PMID: 36334067
- PMCID: PMC10100527
- DOI: 10.1002/glia.24291
Glial progenitor cells of the adult human white and grey matter are contextually distinct
Abstract
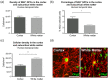
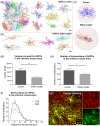
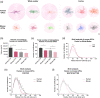
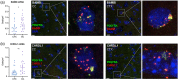
Genomic analyses have revealed heterogeneity among glial progenitor cells (GPCs), but the compartment selectivity of human GPCs (hGPCs) is unclear. Here, we asked if GPCs of human grey and white brain matter are distinct in their architecture and associated gene expression. RNA profiling of NG2-defined hGPCs derived from adult human neocortex and white matter differed in their expression of genes involved in Wnt, NOTCH, BMP and TGFβ signaling, suggesting compartment-selective biases in fate and self-renewal. White matter hGPCs over-expressed the BMP antagonists BAMBI and CHRDL1, suggesting their tonic suppression of astrocytic fate relative to cortical hGPCs, whose relative enrichment of cytoskeletal genes presaged their greater morphological complexity. In human glial chimeric mice, cortical hGPCs assumed larger and more complex morphologies than white matter hGPCs, and both were more complex than their mouse counterparts. These findings suggest that human grey and white matter GPCs comprise context-specific pools with distinct functional biases.
Keywords: NG2; glial heterogeneity; human glial progenitor cells; white and grey matter.
© 2022 The Authors. GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
Dr. Goldman is also a part‐time employee and stock‐holder of Sana Biotechnology, a cell therapy company, and his lab receives sponsored research support from Sana. Maria Joana Osorio is currently employed at Sana. However, none of the work in this report was supported by Sana. No other authors have any known conflicts of interest in regard to this work.
Figures

Similar articles
-
Human ESC-Derived Chimeric Mouse Models of Huntington's Disease Reveal Cell-Intrinsic Defects in Glial Progenitor Cell Differentiation.Cell Stem Cell. 2019 Jan 3;24(1):107-122.e7. doi: 10.1016/j.stem.2018.11.010. Epub 2018 Dec 13. Cell Stem Cell. 2019. PMID: 30554964 Free PMC article.
-
Human iPSC Glial Mouse Chimeras Reveal Glial Contributions to Schizophrenia.Cell Stem Cell. 2017 Aug 3;21(2):195-208.e6. doi: 10.1016/j.stem.2017.06.012. Epub 2017 Jul 20. Cell Stem Cell. 2017. PMID: 28736215 Free PMC article.
-
Young glial progenitor cells competitively replace aged and diseased human glia in the adult chimeric mouse brain.Nat Biotechnol. 2024 May;42(5):719-730. doi: 10.1038/s41587-023-01798-5. Epub 2023 Jul 17. Nat Biotechnol. 2024. PMID: 37460676 Free PMC article.
-
Human Glial Chimeric Mice to Define the Role of Glial Pathology in Human Disease.Methods Mol Biol. 2019;1936:311-331. doi: 10.1007/978-1-4939-9072-6_18. Methods Mol Biol. 2019. PMID: 30820907 Free PMC article. Review.
-
Fate determination of adult human glial progenitor cells.Neuron Glia Biol. 2009 Nov;5(3-4):45-55. doi: 10.1017/S1740925X09990317. Epub 2009 Oct 7. Neuron Glia Biol. 2009. PMID: 19807941 Review.
Cited by
-
Survival of the fittest glia.Nat Biotechnol. 2024 May;42(5):700-702. doi: 10.1038/s41587-023-01944-z. Nat Biotechnol. 2024. PMID: 37640947 No abstract available.
References
-
- Auvergne, R. M. , Sim, F. J. , Wang, S. , Chandler‐Militello, D. , Burch, J. , Al Fanek, Y. , Davis, D., Benraiss, A., Walter, K., Achanta, P., Johnson, M., Quinones‐Hinojosa, A., Natesan, N., Ford, H. L., & Goldman, S. A. (2013). Transcriptional differences between normal and glioma‐derived glial progenitor cells identify a core set of dysregulated genes. CELREP, 3(6), 2127–2141. 10.1016/j.celrep.2013.04.035 - DOI - PMC - PubMed
-
- Belachew, S. , Chittajallu, R. , Aguirre, A. A. , Yuan, X. , Kirby, M. , Anderson, S. , & Gallo, V. (2003). Postnatal NG2 proteoglycan‐expressing progenitor cells are intrinsically multipotent and generate functional neurons. The Journal of Cell Biology, 161(1), 169–186. 10.1083/jcb.200210110 - DOI - PMC - PubMed
-
- Benraiss, A. , Toner, M. J. , Xu, Q. , Bruel‐Jungerman, E. , Rogers, E. H. , Wang, F. , Economides, A. N., Davidson, B. L., Kageyama, R., Nedergaard, M., & Goldman, S. A. (2013). Sustained mobilization of endogenous neural progenitors delays disease progression in a transgenic model of Huntington's disease. Cell Stem Cell, 12(6), 787–799. 10.1016/j.stem.2013.04.014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

