Viscosupplementation for knee osteoarthritis: systematic review and meta-analysis
- PMID: 36333100
- PMCID: PMC9258606
- DOI: 10.1136/bmj-2022-069722
Viscosupplementation for knee osteoarthritis: systematic review and meta-analysis
Abstract
Objective: To evaluate the effectiveness and safety of viscosupplementation for pain and function in patients with knee osteoarthritis.
Design: Systematic review and meta-analysis of randomised trials.
Data sources: Searches were conducted of Medline, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) databases from inception to 11 September 2021. Unpublished trials were identified from the grey literature and trial registries.
Eligibility criteria for study selection: Randomised trials comparing viscosupplementation with placebo or no intervention for knee osteoarthritis treatment.
Main outcome measures: The prespecified primary outcome was pain intensity. Secondary outcomes were function and serious adverse events. Pain and function were analysed as standardised mean differences (SMDs). The prespecified minimal clinically important between group difference was -0.37 SMD. Serious adverse events were analysed as relative risks.
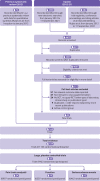
Methods: Two reviewers independently extracted relevant data and assessed the risk of bias of trials using the Cochrane risk of bias tool. The predefined main analysis was based only on large, placebo controlled trials with ≥100 participants per group. Summary results were obtained through a random effects meta-analysis model. Cumulative meta-analysis and trial sequential analysis under a random effects model were also performed.
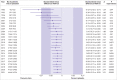
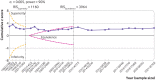
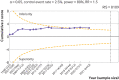
Results: 169 trials provided data on 21 163 randomised participants. Evidence of small study effects and publication biases was observed for pain and function (Egger's tests with P<0.001 and asymmetric funnel plots). Twenty four large, placebo controlled trials (8997 randomised participants) included in the main analysis of pain indicated that viscosupplementation was associated with a small reduction in pain intensity compared with placebo (SMD -0.08, 95% confidence interval -0.15 to -0.02), with the lower bound of the 95% confidence interval excluding the minimal clinically important between group difference. This effect corresponds to a difference in pain scores of -2.0 mm (95% confidence interval -3.8 to -0.5 mm) on a 100 mm visual analogue scale. Trial sequential analysis for pain indicated that since 2009 there has been conclusive evidence of clinical equivalence between viscosupplementation and placebo. Similar conclusions were obtained for function. Based on 15 large, placebo controlled trials on 6462 randomised participants, viscosupplementation was associated with a statistically significant higher risk of serious adverse events than placebo (relative risk 1.49, 95% confidence interval 1.12 to 1.98).
Conclusion: Strong conclusive evidence indicates that viscosupplementation leads to a small reduction in knee osteoarthritis pain compared with placebo, but the difference is less than the minimal clinically important between group difference. Strong conclusive evidence indicates that viscosupplementation is also associated with an increased risk of serious adverse events compared with placebo. The findings do not support broad use of viscosupplementation for the treatment of knee osteoarthritis.
Systematic review registration: PROSPERO CRD42021236894.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Arthritis Society, Canada Research Chairs Programme, National Institute for Health Research, Chevening Scholarship Program for the submitted work. PJ serves as unpaid member of the steering group of trials funded by Appili Therapeutics, Abbot Vascular, and Terumo; he has received research grants to the institution from Appili Therapeutics, and honorariums to the institution for participation in advisory boards or consulting from Amgen, Ava and Fresenius, but has not received personal payments by any pharmaceutical company or device manufacturer. All other authors report no financial relationships with any organisations that might have an interest in the submitted work in the previous three years. All authors report no other relationships or activities that could appear to have influenced the submitted work.
Figures


Similar articles
-
Intra-articular corticosteroid for knee osteoarthritis.Cochrane Database Syst Rev. 2015 Oct 22;2015(10):CD005328. doi: 10.1002/14651858.CD005328.pub3. Cochrane Database Syst Rev. 2015. PMID: 26490760 Free PMC article. Review.
-
Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis.Ann Intern Med. 2012 Aug 7;157(3):180-91. doi: 10.7326/0003-4819-157-3-201208070-00473. Ann Intern Med. 2012. PMID: 22868835 Review.
-
Paracetamol versus placebo for knee and hip osteoarthritis.Cochrane Database Syst Rev. 2019 Feb 25;2(2):CD013273. doi: 10.1002/14651858.CD013273. Cochrane Database Syst Rev. 2019. PMID: 30801133 Free PMC article.
-
Chondroitin for osteoarthritis.Cochrane Database Syst Rev. 2015 Jan 28;1(1):CD005614. doi: 10.1002/14651858.CD005614.pub2. Cochrane Database Syst Rev. 2015. PMID: 25629804 Free PMC article. Review.
-
Surgical interventions for symptomatic mild to moderate knee osteoarthritis.Cochrane Database Syst Rev. 2019 Jul 19;7(7):CD012128. doi: 10.1002/14651858.CD012128.pub2. Cochrane Database Syst Rev. 2019. PMID: 31322289 Free PMC article.
Cited by
-
Prospective bacterial and fungal sources of hyaluronic acid: A review.Comput Struct Biotechnol J. 2022 Nov 10;20:6214-6236. doi: 10.1016/j.csbj.2022.11.013. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36420162 Free PMC article. Review.
-
Intraarticular gold for knee osteoarthritis: An ancillary analysis of biomarkers and outcome of a pilot study.Osteoarthr Cartil Open. 2024 Aug 31;6(4):100514. doi: 10.1016/j.ocarto.2024.100514. eCollection 2024 Dec. Osteoarthr Cartil Open. 2024. PMID: 39291082 Free PMC article. Clinical Trial.
-
Investigating the Anti-Inflammatory, Analgesic, and Chondroprotective Effects of Gynostemma pentaphyllum (Thunb.) Makino in Osteoarthritis: An In Vitro and In Vivo Study.Int J Mol Sci. 2024 Sep 4;25(17):9594. doi: 10.3390/ijms25179594. Int J Mol Sci. 2024. PMID: 39273553 Free PMC article.
-
Inhibitory Effects of Reynoutria japonica Houtt. on Pain and Cartilage Breakdown in Osteoarthritis Based on Its Multifaceted Anti-Inflammatory Activity: An In Vivo and In Vitro Approach.Int J Mol Sci. 2024 Oct 3;25(19):10647. doi: 10.3390/ijms251910647. Int J Mol Sci. 2024. PMID: 39408977 Free PMC article.
-
Safety and efficacy of an allogeneic adipose-derived mesenchymal stem cell preparation in the treatment of knee osteoarthritis: A Phase I/IIa randomised controlled trial.Osteoarthr Cartil Open. 2024 Jul 1;6(3):100500. doi: 10.1016/j.ocarto.2024.100500. eCollection 2024 Sep. Osteoarthr Cartil Open. 2024. PMID: 39161739 Free PMC article.
