Drug Repurposing in Chagas Disease: Chloroquine Potentiates Benznidazole Activity against Trypanosoma cruzi In Vitro and In Vivo
- PMID: 36314800
- PMCID: PMC9664849
- DOI: 10.1128/aac.00284-22
Drug Repurposing in Chagas Disease: Chloroquine Potentiates Benznidazole Activity against Trypanosoma cruzi In Vitro and In Vivo
Abstract
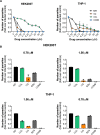
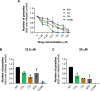
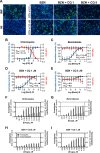
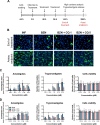
Drug combinations and drug repurposing have emerged as promising strategies to develop novel treatments for infectious diseases, including Chagas disease. In this study, we aimed to investigate whether the repurposed drugs chloroquine (CQ) and colchicine (COL), known to inhibit Trypanosoma cruzi infection in host cells, could boost the anti-T. cruzi effect of the trypanocidal drug benznidazole (BZN), increasing its therapeutic efficacy while reducing the dose needed to eradicate the parasite. The combination of BZN and COL exhibited cytotoxicity to infected cells and low antiparasitic activity. Conversely, a combination of BZN and CQ significantly reduced T. cruzi infection in vitro, with no apparent cytotoxicity. This effect seemed to be consistent across different cell lines and against both the partially BZN-resistant Y and the highly BZN-resistant Colombiana strains. In vivo experiments in an acute murine model showed that the BZN+CQ combination was eight times more effective in reducing T. cruzi infection in the acute phase than BZN monotherapy. In summary, our results demonstrate that the concomitant administration of CQ and BZN potentiates the trypanocidal activity of BZN, leading to a reduction in the dose needed to achieve an effective response. In a translational context, it could represent a higher efficacy of treatment while also mitigating the adverse effects of high doses of BZN. Our study also reinforces the relevance of drug combination and repurposing approaches in the field of Chagas disease drug discovery.
Keywords: Chagas disease drug discovery; benznidazole; chloroquine; drug combination; drug repurposing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Nifurtimox versus benznidazole or placebo for asymptomatic Trypanosoma cruzi infection (Equivalence of Usual Interventions for Trypanosomiasis - EQUITY): study protocol for a randomised controlled trial.Trials. 2019 Jul 15;20(1):431. doi: 10.1186/s13063-019-3423-3. Trials. 2019. PMID: 31307503 Free PMC article.
-
Clomipramine and benznidazole association for the treatment of acute experimental Trypanosoma cruzi infection.Parasitol Int. 2013 Jun;62(3):293-9. doi: 10.1016/j.parint.2013.02.004. Epub 2013 Mar 14. Parasitol Int. 2013. PMID: 23500720
-
Silver and copper-benznidazole derivatives as potential antiparasitic metallodrugs: Synthesis, characterization, and biological evaluation.J Inorg Biochem. 2023 Feb;239:112047. doi: 10.1016/j.jinorgbio.2022.112047. Epub 2022 Nov 8. J Inorg Biochem. 2023. PMID: 36428157
-
Clinical and pharmacological profile of benznidazole for treatment of Chagas disease.Expert Rev Clin Pharmacol. 2018 Oct;11(10):943-957. doi: 10.1080/17512433.2018.1509704. Epub 2018 Sep 19. Expert Rev Clin Pharmacol. 2018. PMID: 30111183 Review.
-
Experimental models in Chagas disease: a review of the methodologies applied for screening compounds against Trypanosoma cruzi.Parasitol Res. 2018 Nov;117(11):3367-3380. doi: 10.1007/s00436-018-6084-3. Epub 2018 Sep 19. Parasitol Res. 2018. PMID: 30232605 Review.
Cited by
-
Synthesis and Anti-Trypanosoma cruzi Activity of New Pyrazole-Thiadiazole Scaffolds.Molecules. 2024 Jul 27;29(15):3544. doi: 10.3390/molecules29153544. Molecules. 2024. PMID: 39124949 Free PMC article.
-
Global Health Priority Box: Discovering Flucofuron as a Promising Antikinetoplastid Compound.Pharmaceuticals (Basel). 2024 Apr 25;17(5):554. doi: 10.3390/ph17050554. Pharmaceuticals (Basel). 2024. PMID: 38794125 Free PMC article.
-
Navigating drug repurposing for Chagas disease: advances, challenges, and opportunities.Front Pharmacol. 2023 Jul 27;14:1233253. doi: 10.3389/fphar.2023.1233253. eCollection 2023. Front Pharmacol. 2023. PMID: 37576826 Free PMC article. Review.
-
Repurposing of rabeprazole as an anti-Trypanosoma cruzi drug that targets cellular triosephosphate isomerase.J Enzyme Inhib Med Chem. 2023 Dec;38(1):2231169. doi: 10.1080/14756366.2023.2231169. J Enzyme Inhib Med Chem. 2023. PMID: 37401012 Free PMC article.
-
Nitazoxanide: A Drug Repositioning Compound with Potential Use in Chagas Disease in a Murine Model.Pharmaceuticals (Basel). 2023 Jun 1;16(6):826. doi: 10.3390/ph16060826. Pharmaceuticals (Basel). 2023. PMID: 37375773 Free PMC article.
References
-
- World Health Organization. 2022. Chagas disease (also known as American trypanosomiasis). World Health Organization, Geneva, Switzerland. https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(america....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

