Myotubularin-Related Protein14 Prevents Neointima Formation and Vascular Smooth Muscle Cell Proliferation by Inhibiting Polo-Like Kinase1
- PMID: 36314496
- PMCID: PMC9673629
- DOI: 10.1161/JAHA.122.026174
Myotubularin-Related Protein14 Prevents Neointima Formation and Vascular Smooth Muscle Cell Proliferation by Inhibiting Polo-Like Kinase1
Abstract
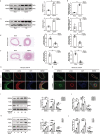
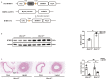
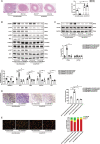
Background Restenosis is one of the main bottlenecks in restricting the further development of cardiovascular interventional therapy. New signaling molecules involved in the progress have continuously been discovered; however, the specific molecular mechanisms remain unclear. MTMR14 (myotubularin-related protein 14) is a novel phosphoinositide phosphatase that has a variety of biological functions and is involved in diverse biological processes. However, the role of MTMR14 in vascular biology remains unclear. Herein, we addressed the role of MTMR14 in neointima formation and vascular smooth muscle cell (VSMC) proliferation after vessel injury. Methods and Results Vessel injury models were established using SMC-specific conditional MTMR14-knockout and -transgenic mice. Neointima formation was assessed by histopathological methods, and VSMC proliferation and migration were assessed using fluorescence ubiquitination-based cell cycle indicator, transwell, and scratch wound assay. Neointima formation and the expression of MTMR14 was increased after injury. MTMR14 deficiency accelerated neointima formation and promoted VSMC proliferation after injury, whereas MTMR14 overexpression remarkably attenuated this process. Mechanistically, we demonstrated that MTMR14 suppressed the activation of PLK1 (polo-like kinase 1) by interacting with it, which further leads to the inhibition of the activation of MEK/ERK/AKT (mitogen-activated protein kinase kinase/extracellular-signal-regulated kinase/protein kinase B), thereby inhibiting the proliferation of VSMC from the medial to the intima and thus preventing neointima formation. Conclusions MTMR14 prevents neointima formation and VSMC proliferation by inhibiting PLK1. Our findings reveal that MTMR14 serves as an inhibitor of VSMC proliferation and establish a link between MTMR14 and PLK1 in regulating VSMC proliferation. MTMR14 may become a novel potential therapeutic target in the treatment of restenosis.
Keywords: MTMR14; PLK1; VSMC; neointima formation; proliferation.
Figures




Similar articles
-
Recent advances of myotubularin-related (MTMR) protein family in cardiovascular diseases.Front Cardiovasc Med. 2024 Mar 11;11:1364604. doi: 10.3389/fcvm.2024.1364604. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38529329 Free PMC article. Review.
-
Salt-Inducible Kinase 3 Promotes Vascular Smooth Muscle Cell Proliferation and Arterial Restenosis by Regulating AKT and PKA-CREB Signaling.Arterioscler Thromb Vasc Biol. 2021 Sep;41(9):2431-2451. doi: 10.1161/ATVBAHA.121.316219. Epub 2021 Jul 1. Arterioscler Thromb Vasc Biol. 2021. PMID: 34196217 Free PMC article.
-
Regulator of G-Protein Signaling 5 Prevents Smooth Muscle Cell Proliferation and Attenuates Neointima Formation.Arterioscler Thromb Vasc Biol. 2016 Feb;36(2):317-27. doi: 10.1161/ATVBAHA.115.305974. Epub 2015 Dec 10. Arterioscler Thromb Vasc Biol. 2016. PMID: 26663397
-
miR-22 Is a Novel Mediator of Vascular Smooth Muscle Cell Phenotypic Modulation and Neointima Formation.Circulation. 2018 Apr 24;137(17):1824-1841. doi: 10.1161/CIRCULATIONAHA.117.027799. Epub 2017 Dec 15. Circulation. 2018. PMID: 29246895 Free PMC article.
-
Correlation between stem cell molecular phenotype and atherosclerotic plaque neointima formation and analysis of stem cell signal pathways.Front Cell Dev Biol. 2023 Jan 12;11:1080563. doi: 10.3389/fcell.2023.1080563. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36711040 Free PMC article. Review.
Cited by
-
Effect of acidosis on adipose-derived stem cell impairment and gene expression.Regen Ther. 2024 Feb 5;25:331-343. doi: 10.1016/j.reth.2024.01.010. eCollection 2024 Mar. Regen Ther. 2024. PMID: 38333090 Free PMC article.
-
Recent advances of myotubularin-related (MTMR) protein family in cardiovascular diseases.Front Cardiovasc Med. 2024 Mar 11;11:1364604. doi: 10.3389/fcvm.2024.1364604. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38529329 Free PMC article. Review.
References
-
- DALYs GBD and Collaborators H . Global, regional, and national disability‐adjusted life‐years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1260–1344. doi: 10.1016/S0140-6736(17)32130-X - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

