Collagen peptide promotes DSS-induced colitis by disturbing gut microbiota and regulation of macrophage polarization
- PMID: 36313077
- PMCID: PMC9608506
- DOI: 10.3389/fnut.2022.957391
Collagen peptide promotes DSS-induced colitis by disturbing gut microbiota and regulation of macrophage polarization
Abstract
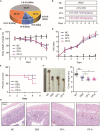
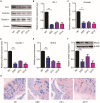
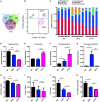
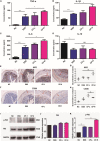
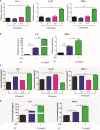
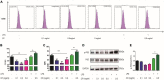
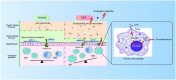
Ulcerative colitis (UC) is an inflammatory bowel disease caused by mucosal immune system disorder, which has increased steadily all over the world. Previous studies have shown that collagen peptide (CP) has various beneficial biological activities, it is not clear whether the effect of CP on UC is positive or negative. In this study, 2.5% dextran sulfate sodium (DSS) was used to establish acute colitis in mice. Our results suggested that CP supplementation (200, 400 mg/kg/day) promoted the progression of colitis, increased the expression of inflammatory factors and the infiltration of colonic lamina propria macrophages. Gut microbiota analysis showed the composition changed significantly and inflammation promoted bacteria was after CP treatment. Meanwhile, the effect of CP on macrophage polarization was further determined in Raw264.7 cell line. The results showed that CP treatment could increase the polarization of M1 macrophages and promote the expression of inflammatory factors. In conclusion, our results showed that CP treatment could disrupt the gut microbiota of host, promote macrophage activation and aggravate DSS-induced colitis. This may suggest that patients with intestinal inflammation should not take marine derived CP.
Keywords: DSS-induced colitis; collagen peptide; gut microbiota; intestinal barrier; macrophage polarization.
Copyright © 2022 Li, Cui, Feng, Yu, Shao, He and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Sea Cucumber Peptides Ameliorate DSS-Induced Ulcerative Colitis: The Role of the Gut Microbiota, the Intestinal Barrier, and Macrophage Polarization.Nutrients. 2023 Nov 17;15(22):4813. doi: 10.3390/nu15224813. Nutrients. 2023. PMID: 38004208 Free PMC article.
-
Cod (Gadus) skin collagen peptide powder reduces inflammation, restores mucosal barrier function, and inhibits fibrosis in dextran sodium sulfate-induced colitis in mice.J Ethnopharmacol. 2023 Nov 15;316:116728. doi: 10.1016/j.jep.2023.116728. Epub 2023 Jun 3. J Ethnopharmacol. 2023. PMID: 37277083
-
Huangqin Decoction ameliorates ulcerative colitis by regulating fatty acid metabolism to mediate macrophage polarization via activating FFAR4-AMPK-PPARα pathway.J Ethnopharmacol. 2023 Jul 15;311:116430. doi: 10.1016/j.jep.2023.116430. Epub 2023 Mar 28. J Ethnopharmacol. 2023. PMID: 36997133
-
CP-25 exerts therapeutic effects in mice with dextran sodium sulfate-induced colitis by inhibiting GRK2 translocation to downregulate the TLR4-NF-κB-NLRP3 inflammasome signaling pathway in macrophages.IUBMB Life. 2021 Dec;73(12):1406-1422. doi: 10.1002/iub.2564. Epub 2021 Oct 19. IUBMB Life. 2021. PMID: 34590407
-
2,3,5,4'-Tetrahydroxystilbene-2-O-β-D-glucoside, a major bioactive component from Polygoni multiflori Radix (Heshouwu) suppresses DSS induced acute colitis in BALb/c mice by modulating gut microbiota.Biomed Pharmacother. 2021 May;137:111420. doi: 10.1016/j.biopha.2021.111420. Epub 2021 Feb 23. Biomed Pharmacother. 2021. PMID: 33761623
Cited by
-
Genistein alleviates dextran sulfate sodium-induced colitis in mice through modulation of intestinal microbiota and macrophage polarization.Eur J Nutr. 2024 Aug;63(5):1877-1888. doi: 10.1007/s00394-024-03391-1. Epub 2024 Apr 9. Eur J Nutr. 2024. PMID: 38592519
-
Glycated Casein by TGase-Type Exerts Protection Potential against DSS-Induced Colitis via Inhibiting TLR4/NF-κB Signaling Pathways in C57BL/6J Mice.Foods. 2023 Sep 14;12(18):3431. doi: 10.3390/foods12183431. Foods. 2023. PMID: 37761139 Free PMC article.
-
C-Phycocyanin Ameliorates the Senescence of Mesenchymal Stem Cells through ZDHHC5-Mediated Autophagy via PI3K/AKT/mTOR Pathway.Aging Dis. 2023 Aug 1;14(4):1425-1440. doi: 10.14336/AD.2023.0121. Aging Dis. 2023. PMID: 37163424 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

