Biting the hand that feeds: Metabolic determinants of cell fate during infection
- PMID: 36311735
- PMCID: PMC9614662
- DOI: 10.3389/fimmu.2022.923024
Biting the hand that feeds: Metabolic determinants of cell fate during infection
Abstract
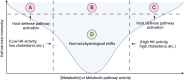
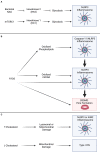
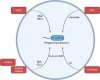
Metabolic shifts can occur in cells of the innate immune system in response to microbial infection. Whether these metabolic shifts benefit host defense and propagation of an immune response appears to be context dependent. In an arms race, host-adapted microbes and mammalian cells vie for control of biosynthetic machinery, organelles, and metabolites. Herein, we discuss the intersection of host metabolism and cell-intrinsic immunity with implications for cell fate during infection. Sensation of microbial ligands in isolation results in host metabolic shifts that imbues normal innate immune function, such as cytokine secretion. However, living microbes have an arsenal of effectors and strategies to subvert cell-intrinsic immune responses by manipulating host metabolism. Consequently, host metabolism is monitored as an indicator of invasion or manipulation by a pathogen, primarily through the actions of guard proteins and inflammasome pathways. In this review, we frame initiation of cell-intrinsic immunity in the context of host metabolism to include a physiologic "Goldilocks zone" of allowable shifts with guard circuits monitoring wide perturbations away from this zone for the initiation of innate immune responses. Through comparison of studies with purified microbial ligands, dead microbes, and live pathogens we may begin to understand how shifts in metabolism determine the outcome of host-pathogen interactions.
Keywords: cell fate decisions; cell-intrinsic immunity; guard theory; host-pathogen arms race; inflammasome; inflammation; metabolism; pathogenesis.
Copyright © 2022 Fraschilla and Evavold.
Conflict of interest statement
The reviewers MG and XZ declared a shared parent affiliation with the authors to the handling editor at the time of review.
Figures
Similar articles
-
Vying for the control of inflammasomes: The cytosolic frontier of enteric bacterial pathogen-host interactions.Cell Microbiol. 2020 Apr;22(4):e13184. doi: 10.1111/cmi.13184. Cell Microbiol. 2020. PMID: 32185892 Free PMC article. Review.
-
Inflammasomes on the Crossroads of Innate Immune Recognition and Metabolic Control.Cell Metab. 2017 Jul 5;26(1):71-93. doi: 10.1016/j.cmet.2017.06.018. Cell Metab. 2017. PMID: 28683296 Review.
-
Inflammasomes, Autophagy, and Cell Death: The Trinity of Innate Host Defense against Intracellular Bacteria.Mediators Inflamm. 2019 Jan 8;2019:2471215. doi: 10.1155/2019/2471215. eCollection 2019. Mediators Inflamm. 2019. PMID: 30728749 Free PMC article. Review.
-
Pathogen subversion of cell-intrinsic innate immunity.Nat Immunol. 2007 Nov;8(11):1179-87. doi: 10.1038/ni1528. Nat Immunol. 2007. PMID: 17952043 Review.
-
Inflammasomes: Threat-Assessment Organelles of the Innate Immune System.Immunity. 2019 Oct 15;51(4):609-624. doi: 10.1016/j.immuni.2019.08.005. Epub 2019 Aug 28. Immunity. 2019. PMID: 31473100 Free PMC article. Review.
Cited by
-
Hepatic insulin synthesis increases in rat models of diabetes mellitus type 1 and 2 differently.PLoS One. 2023 Nov 29;18(11):e0294432. doi: 10.1371/journal.pone.0294432. eCollection 2023. PLoS One. 2023. PMID: 38019818 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

